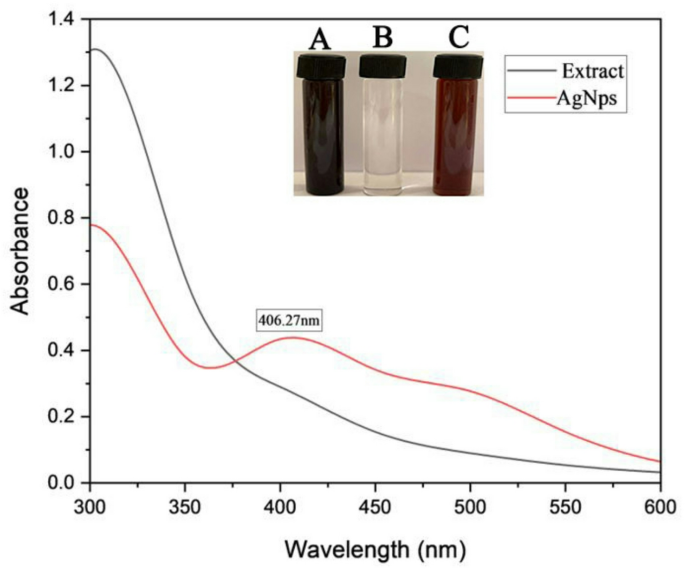
The green synthesis of AgNPs was confirmed by a distinct color change, serving as a visual indicator of At-AgNPs formation. In this context, the brown coloration results from the surface plasmon resonance (SPR) effect, a distinctive optical feature exhibited by AgNPs. When AgNPs are formed, their free electrons oscillate collectively upon interaction with light, leading to the absorption of specific wavelengths and the reflection of others, resulting in the observed brown color17. The aqueous extract of Alkanna tinctoria roots comprises several phytochemicals, including alkaloids, flavonoids, phenolic compounds, and terpenoids, which function as reducing agents18. These compounds provide electrons to Ag⁺ ions in the AgNO₃ solution, thus reducing them to metallic silver (Ag⁰). Moreover, the phytochemicals serve as capping agents, stabilizing the biosynthesized AgNPs and inhibiting their aggregation. The biosynthesized AgNPs exhibited a characteristic SPR peak at 406.27 nm, consistent with typical SPR behavior of silver nanoparticles. These findings align with previous reports using Hagenia abyssinica leaf extract, which demonstrated a similar SPR peak at 406 nm19. Moreover, TEM analysis revealed spherical AgNPs with a mean particle diameter of 19.91 nm and a size distribution ranging from 5 to 30 nm. Previous research revealed that TEM analysis showed the spherical shape of AgNPs synthesized from Barleria prattensis, with a mean size of 28.57 nm ± 7.8. This organic capping layer improves the stabilization of the Bp-AgNPs by effectively inhibiting particle agglomeration20. Furthermore, the AgNPs synthesized from three Sideritis species exhibited a spherical and monodispersed morphology, with mean size ranging between 22 and 26 nm21. Collectively, our findings demonstrate a significantly smaller particle size compared to those reported in prior studies, underscoring the superior efficiency of the green synthesis method employed in the current investigation. Moreover, green synthesis using Alkanna tinctoria root extract provides a safer alternative, employing natural phytochemicals from the extract as reducing and stabilizing agents, unlike chemical synthesis methods, which require toxic reducing and stabilizing agents that can leave hazardous residues on the nanoparticles and limit their practical use22. Moreover, the current study achieved smaller nanoparticle sizes (19.91 nm) compared to those reported by Ashraf et al. (2015), who employed a chemical synthesis method using sodium citrate solution as a reducing agent to produce AgNPs ranging from 42 to 58 nm23. Moreover, other study reported the chemical method of AgNPs synthesis using sodium borohydride resulting in particle sizes ranged from 15 to 85 nm in diameter according to TEM results24. Collectively, these comparisons demonstrate that our green synthesis approach not only provides an environmentally safe alternative but also yields significantly smaller nanoparticles, highlighting the superior efficiency of this method.
On the other hand, EDX analysis confirmed the elemental composition of the nanoparticles, with silver (Ag) dominating at 79.24% of the total mass. Additional signals revealed carbon (6.03%) and oxygen (8.44%), likely originating from the plant-derived capping agents. The presence of carbon (C) at 6.03% and oxygen (O) at 8.44% suggests the involvement of organic compounds derived from the root extract, likely acted as both stabilizing and reducing agents during the nanoparticle synthesis25. Furthermore, the presence of chlorine (Cl⁻) ions at 6.29% is consistent with prior research, which has established that Cl⁻ ions are abundant in plants and play a vital role in maintaining cellular homeostasis and facilitating photosynthetic processes. This may account for the prominent Cl⁻ peak observed in the EDX spectrum of the biogenic AgNPs26. The total mass percentage of 100.00% confirmed the precision and reliability of the EDX measurements. Collectively, these findings underscore the efficacy of the root extract in the synthesis and stabilization of AgNPs, while also highlighting the contribution of organic capping agents and plant-derived ions to the nanoparticles’ structural integrity and dispersion. Moreover, FTIR analysis demonstrated the presence of different capping agents over the surface of AgNPs as phenolic, alcoholic, aldehydes, and amines. Interestingly, the XRD pattern’s distinct peaks at 2θ = 38.515° (111), 44.540° (200), 64.566° (220), 77.508° (311), and 81.762° (222) confirm the face-centered cubic (fcc) crystalline structure of At-AgNPs, matching standard metallic silver (JCPDS 04-0782). The existence of these peaks verifies the successful biosynthesis of crystalline AgNPs, with the measured 2θ values and related lattice planes closely matching the standard diffraction pattern for metallic silver, hence affirming the crystalline nature of At-AgNPs27. The low PDI value of At-AgNPs (0.202) confirms a monodisperse, homogeneous sample28. However, the observed size discrepancy between DLS (120.6 nm) and TEM arises because DLS measures nanoparticles with their hydrate layer and phytochemical capping agents, while TEM provides exact physical dimensions29. The zeta potential (− 23.34 mV) indicates moderate colloidal stability, attributed to negatively charged functional groups from A. tinctoria root extract adsorbed on the nanoparticle surface30.
The tested Candida strains exhibited distinct resistance patterns when evaluated for antifungal susceptibility using the disk diffusion method. C. albicans and C. glabrata demonstrated resistance to terbinafine, while C. auris and C. krusei were resistant to fluconazole. Additionally, C. glabrata showed resistance to itraconazole, C. auris to natamycin and tioconazole, and C. krusei to tioconazole. Among all the antifungal agents tested, nystatin was the most effective against the different Candida species, showing the highest inhibitory activity. The resistance patterns observed in this study align with known mechanisms of antifungal resistance. Terbinafine resistance in C. albicans and C. glabrata likely results from mutations in the ERG1 gene or overexpression of efflux pumps such as CDR1 and CDR2, which disrupt ergosterol biosynthesis by targeting squalene epoxidase31. Similarly, fluconazole resistance in C. auris and C. krusei can be attributed to ERG11 mutations, increased efflux pump activity (MDR1, CDR1), or, in the case of C. krusei, inherent structural differences in the ERG11-encoded enzyme32. The widespread resistance to azoles (fluconazole, itraconazole) and polyenes (natamycin) highlights the growing challenge in treating candidal infections, particularly with the rising prevalence of multidrug-resistant strains like C. auris. While nystatin demonstrated strong efficacy, its clinical use is limited due to toxicity concerns, emphasizing the need for alternative antifungal agents. In this context, silver nanoparticles (AgNPs) synthesized using Alkanna tinctoria root extract were investigated as a potential solution. Their unique mechanism of action, distinct from conventional antifungals, may help overcome existing resistance mechanisms, offering a promising therapeutic alternative that warrants further exploration. Remarkably, the results demonstrated that while the Alkanna tinctoria root extract itself showed no antifungal activity (0.00 ± 0.00 mm inhibition zones across all Candida species), the AgNPs synthesized from the extract exhibited significant inhibitory effects. The AgNPs (1.0 mg) produced measurable inhibition zones ranging from 9.69 ± 0.93 mm (C. tropicalis) to 15.46 ± 0.68 mm (C. parapsilosis), with particularly strong activity against C. parapsilosis. The positive control (terbinafine 30 µg) showed variable efficacy, with high activity against C. tropicalis (22.55 ± 0.56 mm) and C. parapsilosis (38.42 ± 1.16 mm) but no effect on C. glabrata (0.00 ± 0.00 mm), consistent with earlier resistance patterns.
Our findings represented significant advancements over previous reports of green-synthesized AgNPs, particularly in nanoparticle characteristics and antifungal efficacy. While Arsène et al. successfully produced cubic AgNPs with a hydrodynamic diameter of 80.31 ± 10.03 nm and an SPR peak at 429.83 nm using Aloe vera extract, the biosynthesized A. tinctoria-derived AgNPs demonstrated superior physicochemical properties. The smaller particle size (19.91 nm by TEM versus 80.31 nm by DLS in previous work) enabled better membrane penetration and cellular uptake33. Furthermore, At-AgNPs exhibited broader antifungal activity, including against resistant strains like C. glabrata (10.25 ± 0.40 mm) and C. auris (12.36 ± 0.29 mm), whereas prior studies only reported dose-dependent efficacy against C. albicans isolates (inhibition zones: 0–22 mm). Most notably, we demonstrated the enhanced synergistic effects with conventional antifungals. In this regard, the biogenic At-AgNPs improved clotrimazole efficacy by approximately 10% (37.28 mm versus 33.84 mm alone), while previous research focused solely on the antifungal activity of AgNPs alone. Moreover, a previous report have demonstrated the antifungal activity of AgNPs synthesized using date palm leaf extract, reporting inhibition zone diameters (IZD) of 18 mm and 21 mm against Aspergillus niger and C. albicans, respectively34. However, this study didn’t examine the synergistic potential of AgNPs with conventional antifungals. Our study addresses this gap by evaluating the ability of AgNPs to enhance the efficacy of standard antifungal agents.
The anticandidal mechanism of At-AgNPs employs a multimodal strategy that disrupts the structural and functional integrity of candidal cells, resulting in their lysis. In this context, AgNPs exhibit fungicidal effects mainly by interacting with the fungal cell membrane, disrupting its integrity, increasing permeability, and inducing leaking of internal contents, finally leading to cell lysis35. Additionally, AgNPs stimulate the production of reactive oxygen species (ROS) in cells, resulting in oxidative stress that damages lipids, proteins, and DNA, hence compromising cellular homeostasis and metabolic functions36,37. The At- AgNPs block critical enzymatic processes by attaching to sulphur- and phosphorus-containing groups in proteins and DNA, hence affecting functions such as cellular respiration, energy generation, and DNA replication38. Furthermore, AgNPs may infiltrate the cell wall and nucleus, resulting in DNA damage, strand breakage, and the inhibition of replication and transcription, so obstructing cell division39. Mitochondrial dysfunction represents a significant pathway, since AgNPs interfere with the electron transport chain, diminishing ATP synthesis and intensifying ROS generation40.
The emergence of antifungal-resistant strains underscores the urgent need for innovative combinatorial strategies. Leveraging the inherent antifungal efficacy of At-AgNPs, we systematically assessed their synergistic interactions with conventional antifungals against the tested Candida strains. This study’s findings illustrate the capability of At-AgNPs to augment the antifungal efficacy of conventional antifungal drugs, such as clotrimazole, fluconazole, nystatin, and terbinafine, against different candidal pathogens.
The most significant synergistic impact was seen with the combination of At-AgNPs and clotrimazole against C. albicans, shown by the substantial increase in the inhibition zone diameter (37.28 mm for the combination compared to 33.84 mm for clotrimazole alone). This improvement may be ascribed to the combined mechanistic effects of AgNPs and clotrimazole. Silver nanoparticles are recognized for their capability to damage fungal cell membranes via ROS generation and the compromise of candidal membrane integrity, while clotrimazole inhibits the manufacture of ergosterol, a vital constituent of candidal cell membranes41. The synergistic action probably increases membrane permeability and cellular damage, resulting in heightened antifungal activity. The synergistic impact seen with the combination of nystatin and terbinafine may be elucidated by the complimentary mechanisms of both drugs. Nystatin interacts with ergosterol, forming holes in the fungal membrane, whilst AgNPs further compromise the membrane integrity42. Terbinafine, an inhibitor of squalene epoxidase, interrupts ergosterol production43. When used in conjunction with AgNPs, it may enhance antifungal efficacy by simultaneously targeting membrane integrity and ergosterol synthesis44.
The variability in synergistic patterns among different Candida strains, such as C. albicans, C. krusei, and C. auris, can be attributed to differences in their intrinsic resistance mechanisms and membrane compositions. For instance, C. krusei and C. auris are known for their inherent fluconazole resistance due to alterations in drug target enzymes (e.g., lanosterol 14α-demethylase) and efflux pump overexpression. However, the combination of antifungals with AgNPs demonstrated improved antifungal activity against resistant strains, likely mediated by enhanced membrane permeability and drug internalization45. This work highlights the synergistic impact of combining AgNPs with conventional antifungals to address resistant fungal infections, especially when monotherapy is inadequate.
While this study demonstrates the promising antifungal synergy of At-AgNPs, several limitations should be noted. The lack of cytotoxicity evaluation against mammalian cells limits assessment of therapeutic selectivity, and physiological relevance requires verification through in vivo studies examining pharmacokinetics and safety profiles. Subsequent research efforts should focus on three key areas: establishing biosafety parameters through cytotoxicity profiling, validating efficacy in animal models, and investigating biofilm disruption potential given its clinical significance in Candida infections. Deeper mechanistic exploration of molecular targets, including membrane proteins and oxidative stress pathways, would further optimize these combinatorial approaches for clinical translation.
This study highlights the successful green synthesis of small (19.91 nm), monodisperse (PDI: 0.202) AgNPs utilizing A. tinctoria aqueous extract, which exhibit superior antifungal activity particularly against resistant Candida strains as C. glabrata. The synergistic activity of AgNPs with conventional antifungals (clotrimazole, nystatin, terbinafine) demonstrated a breakthrough in combating resistance. For instance, combining AgNPs with clotrimazole increased inhibition zones by ~ 10% against C. albicans, leveraging dual mechanisms: AgNPs disrupt membranes via ROS, while antifungals target ergosterol synthesis. These findings offer a promising solution to the growing crisis of antifungal resistance, providing a basis for developing novel combination therapies for fungal infections, particularly in immunocompromised patients. Future clinical studies should validate these results in vivo to accelerate translation into treatment protocols.