Proteins are typically viewed as molecular machines. When properly folded, a protein can perform its desired function. However, many proteins are modified during production to carry carbohydrate side chains known as glycans. These sugar structures rarely appear in textbook protein depictions but play decisive roles in health and disease. The ABO blood-group system offers an intuitive example: the A, B and O types differ only in the terminal sugars decorating red blood cell membrane glycoproteins, and that small change alone determines transfusion compatibility. Similar glycan codes also guide other phenomena in biology, such as immune surveillance, viral attachment and cell migration throughout the body.
What are glycans?
Glycans can enhance, dampen or redirect protein activity. Glycan modification can alter size, charge and geometry, shield an enzyme from degradation, help a hormone find its receptor or steer an antibody toward a specific immune response. Yet the production of glycans on proteins is poorly understood, as the glycan attachment (glycosylation) process is not template-driven. Unlike DNA replication or protein synthesis, which follow a strict nucleotide or amino acid sequence, glycan assembly relies on a network of enzymes in the endoplasmic reticulum and Golgi apparatus that add or trim sugars stepwise. As a result, glycan patterns are heterogeneous and sensitive to their environment and cell environmental stresses.
Nearly every secreted or membrane-bound human protein carries glycans as a post-translational modification. Many therapeutic proteins are manufactured with carefully tuned glycosylation to ensure potency and safety. For example, cancer-fighting monoclonal antibodies depend on their attached sugars to efficiently recruit immune cells, and recombinant clotting factors rely on complex branching to achieve proper half-life in circulation.
Because glycans influence so many facets of protein behavior, the ability to analyze and control them has become important in modern biopharmaceutical development. Here, we explain glycans, their structure, their contribution to disease and the analytical techniques used to study them.
A quick glyco-history
The story of glycans begins in classic chemistry. Emil Fischer’s 1891 stereochemical proof of glucose and subsequent findings of its isomers gave scientists the blueprint of how sugars can vary in three-dimensional space, establishing the nomenclature of the carbohydrate building blocks we still use today.1 Fischer also showed that sugars can react with alcohols to produce sugar alcohol conjugates known as glycosides, laying the foundation for understanding glycosylation.
A decade later, Karl Landsteiner’s 1901 paper on blood-cell agglutination revealed that terminal sugars on red blood cell glycoproteins generate the A, B and O blood groups, proof that carbohydrates can dictate life-or-death compatibility in humans.2 The first clear glimpse of sugar bonded to a protein came in 1938 when Hans Neuberger reported hexosamines and mannose attached to ovalbumin, coining the term glycoprotein.3 That finding transformed our understanding of proteins from simple amino-acid-based structures into composite molecules whose sugar attachments matter as much as the peptide backbone.
Fast-forward to biopharma’s early years, by the 1980s, researchers knew that extra sialic acid on recombinant human erythropoietin prolonged the hormone’s serum half-life and boosted in vivo potency.4 This concept led to the long-acting drug darbepoetin alfa. This was a historic moment. Change one sugar, and you change a drug.
Over the past 120 years, glycans have moved from ambiguity to a critical quality attribute (CQA) for modern biologics. They are covalent sugar chains, most commonly N-linked, O-linked or, less often, lipid-anchored, producing families of glycans with different variations known as “glycoforms” with distinct biological effects.
Although mammalian cells employ only a small alphabet of sugars, namely glucose, galactose, mannose, fucose, N-acetyl-glucosamine (GlcNAc), N-acetyl-galactosamine (GalNAc) and the sialic acid N-acetyl-neuraminic acid, they arrange these units into a remarkable variety of carbohydrate chains (Figure 1). A glycosidic bond links the anomeric carbon of one sugar to a hydroxyl group of the next. Its stereochemistry (α or β) describes the bond and the participating carbon atoms. For example, Galβ1-4GlcNAc tells us that galactose is in the beta configuration and attached from its C1 to the C4 of GlcNAc. Branch points arise when a monosaccharide forms two glycosidic bonds, creating antennae from a common core.
Two attachment chemistries dominate biologics: N-linked and O-linked glycans. N-linked glycans are attached to the side-chain nitrogen of an asparagine that sits within the amino acid consensus Asn-X-Ser/Thr motif, where X is any amino acid except proline.5 During translation, a lipid-linked pentasaccharide core, Man₃GlcNAc₂, is transferred en bloc to the growing polypeptide in the endoplasmic reticulum. Subsequent trimming and rebuilding in the Golgi sorts this core into three architectural families. High-mannose structures retain several extra mannoses and lack outer-arm GlcNAc. Hybrid glycans remodel only one antenna, leaving the other mannose rich. Complex glycans remodel both arms with GlcNAc, add β-linked galactose and often cap with a terminal sialic acid; nearly all human N-glycans carry a single core fucose off the innermost GlcNAc. The degree of branching can reach two, three or four antennae, and each extra branch can increase solubility, adjust receptor affinity or prolong serum half-life.6 For antibodies, deleting the core fucose alone can double antibody-dependent cellular cytotoxicity by increasing the affinity of IgG Fc for FcγRIIIa,7 a principle that underpins several next-generation antibodies.
O-linked glycans follow a different logic. Once a protein reaches the Golgi, a GalNAc transferase primes the hydroxyl oxygen of serine or threonine, after which various glycosyl-transferases extend the chain one sugar at a time. Early glycochemists grouped these mucin-type O-glycans into a handful of core motifs. Core 1 consists of Galβ1-3GalNAc and is the most widespread. Core 2 branches the primer by adding GlcNAcβ1-6 to the GalNAc, creating a scaffold that can accept repeating Gal-GlcNAc disaccharides. Cores 3 and 4, found mainly in the gut epithelium, invert the branching order with GlcNAc and create linear or branched chains contributing to the protective mucus barrier. Depending on the protein context, an O-glycan may remain a simple sialylated disaccharide, as seen on many circulating hormones, or extend into the densely packed sugar brush that shields mucins from proteases. Site selection relies more on local protein conformation than on a fixed amino acid motif, explaining the patchy clusters of O-glycans that appear in intrinsically disordered regions of proteins such as mucin 1.8
N-linked and O-linked glycans share the same sugar alphabet but diverge in their core scaffolds, branching logic and biosynthetic timing. N-glycans expand from a conserved base and are diversified broadly by antenna length and terminal capping. In contrast, O-glycans begin with several small cores that may remain concise or polymerize into extended chains. Even minute deviations in glycan profiles serve as red flags during process development. For example, Afucosylated Fc N-glycans sharpen the tumor-killing power of therapeutic IgG,9 controlled α2-6 sialylation extends the circulating half-life of clotting factors10 and deliberate exposure of the truncated Core 1 T antigen on tumor mucins supplies a neo-epitope for glycopeptide vaccines.11
Figure 1. The monosaccharide building blocks involved in glycosylation and example structures of O-linked vs N-linked glycans. Credit: Technology Networks.
The role of glycosylation in disease and biotherapeutic development
Glycans guide nascent proteins toward correct folding, protect them from proteolysis and serve as recognition motifs for lectins and other carbohydrate‑binding receptors on neighboring cells. Because glycosylation depends on the local activities of glycosyltransferases and glycosidases, the resulting patterns are exquisitely sensitive to cell type, nutrient availability and environmental stress.
In healthy tissues, this dynamic regulation ensures proper cell-cell adhesion, immune surveillance and receptor signaling; in the bloodstream,12 terminal sialic acids prevent rapid clearance by liver asialoglycoprotein receptors, extending a glycoprotein’s half‑life.
In disease, cells often hijack glycosylation to their advantage. Cancer cells universally display aberrant glycans: they over‑branch N‑linked structures, elevate core fucosylation and hypersialylate their surfaces.13 Branched N‑glycans on adhesion molecules weaken cell-cell contacts and promote invasion. At the same time, excess sialic acid engages inhibitory Siglec receptors on natural killer cells and macrophages, suppressing anti‑tumor immunity. Fucosylated Lewis antigens facilitate tumor arrest on endothelium and metastasis. In neurodegenerative conditions, age‑related shifts toward bisected and branched N‑glycans have been detected in cerebrospinal fluid, and altered O‑GlcNAcylation of Tau and amyloid precursor protein influences aggregation and clearance in Alzheimer’s disease.14 Autoimmune disorders also carry distinct glycan signatures: patients produce IgG with reduced galactosylation and sialylation on the Fc N‑glycan, exposing pro‑inflammatory epitopes that amplify complement activation and Fcγ receptor‑mediated cytotoxicity.15
These mechanistic insights have inspired glycoengineering strategies in biopharmaceutical development. Recombinant erythropoietin was modified with additional N‑glycosylation sites and enhanced sialylation to slow renal filtration and avoid hepatic clearance, extending its half‑life.16 Monoclonal antibodies routinely exploit afucosylated Fc glycans to boost antibody‑dependent cellular cytotoxicity against cancer targets without altering antigen specificity. Enzyme replacement therapies for lysosomal storage diseases are produced with exposed mannose or mannose‑6‑phosphate termini, ensuring efficient uptake via macrophage mannose receptors.17 Biologics can be designed with optimized stability, targeting and immune‑modulating properties by harnessing the sugar codes underlying the disease.
What is glycan analysis?
Glycan analysis is the process of characterizing carbohydrate chains attached to proteins and lipids. In practice, this involves many techniques, including sample workup and interpretation through analytical methods such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS). In biologic development, glycan analysis is indispensable. Regulatory agencies treat glycosylation as a CQA since glycan composition influences a drug’s stability, efficacy and immunogenicity. Consistent glycosylation profiles are required to ensure batch‑to‑batch reproducibility.
Glycan analysis of biopharmaceuticals
No single method can capture every aspect of glycosylation. Combined, complementary techniques are used to get a holistic understanding of glycans, including their detection, structures, site specificity, monosaccharide composition and linkage positions (Figure 2). Below are a few methods to help decipher key methodologies to analyze glycosylation.
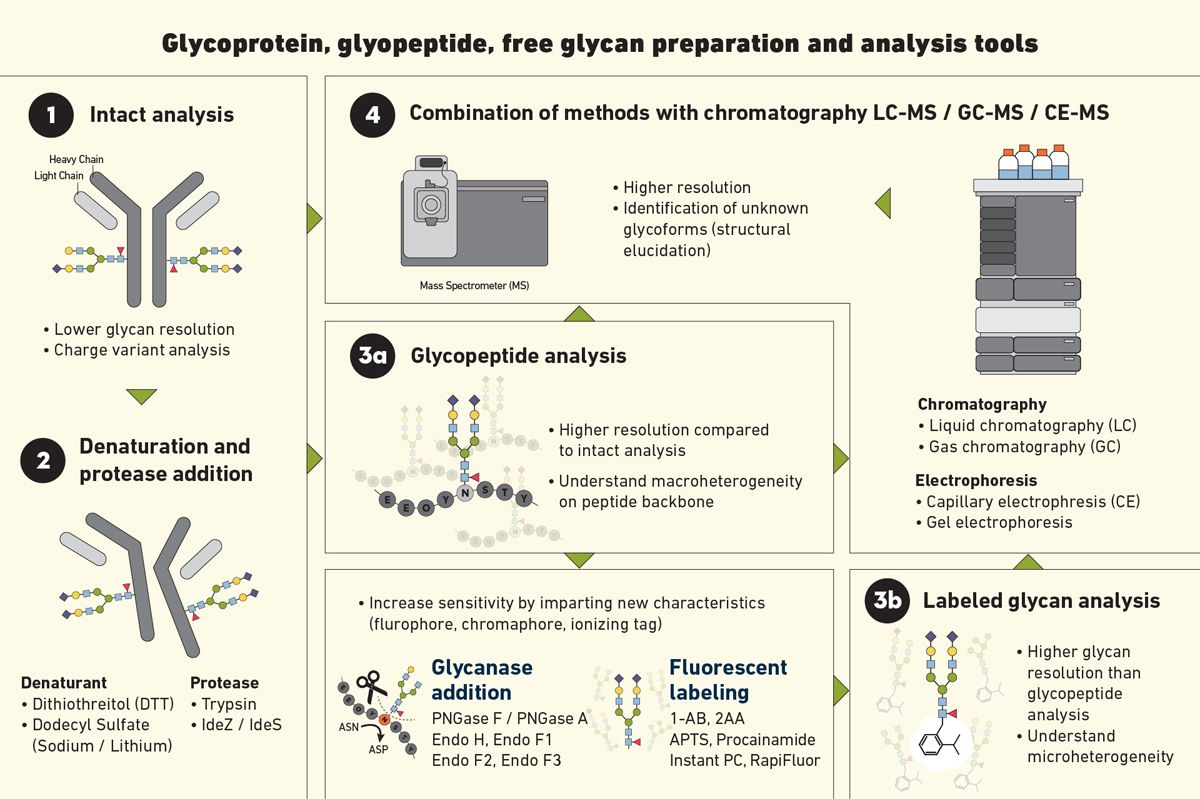
Figure 2. Examples of some of the methods used for glycan analysis, including intact analysis, glycopeptide analysis and labeled glycan analysis. Credit: Technology Networks.
Detection of glycans
straightforward methodology to capture and understand glycans is to use carbohydrate-binding proteins, called lectins, that bind to the terminal sugar of the protein. A typical workflow uses a lectin array in which a panel of lectins is immobilized on a slide or biosensor surface, and fluorescently labeled glycoproteins are applied. Each lectin spot captures glycoproteins displaying its cognate sugar, and fluorescence intensity at each spot indicates the presence and relative abundance of the terminal sugar. Glycosylation also alters protein size and hydrodynamic radius. Hence, on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, heavily sialylated or branched glycoforms migrate more slowly, and on size‑exclusion chromatography, they elute earlier. Even marginal changes, such as adding or removing core fucose, can produce detectable electrophoretic mobility or retention shifts, providing an orthogonal method for altered glycosylation determination.
Glycan structure determination
The process of glycan structure determination typically involves the following:
- Glycan structures are usually identified after enzymatic or chemical release from their protein backbone. N-glycans are commonly released using an enzyme called Peptide:N-glycosidase F (PNGase F). O-glycans typically are removed chemically by β-elimination.
- The released glycans are labeled with a fluorescent tag, like 2-aminobenzamide, for improved chromatographic separation and detection.
- For detection, HPLC equipped with a hydrophilic interaction chromatography column separates labeled glycans based on polarity and branching.
- For further determination, matrix-assisted laser desorption/ionization time of flight mass spectrometry can be used to identify glycan compositions by mass.
Typically, a workflow would use the methodologies in steps 3 and 4 together to gain more insight into the glycan structure by liquid chromatography-mass spectrometry (LC-MS). For further structural resolution, tandem MS (MS/MS) fragmentation or enzymatic treatments (exoglycosidase sequencing) can be used for branching and sequence details.
Intact glycoprotein analysis
Analyzing glycoproteins in their intact form provides a broad overview of their glycosylation profiles, albeit less sensitively. Electrospray ionization-mass spectrometry is frequently used to distinguish glycoforms. Intact analysis quickly identifies major glycan variants without prior enzymatic treatment, which is helpful for rapid screening and quality control. For large proteins like antibodies, enzymatic fragmentation using proteases such as IdeS simplifies mass analysis by cleaving the antibody at the hinge region, isolating smaller glycopeptide fragments more amenable to accurate glycan profiling by LC-MS. Other techniques for intact glycoprotein analysis include isoelectric focusing or capillary electrophoresis, which can identify glycoform distributions based on charge difference. These are particularly useful for sialylated proteins, as sialic acid contains charge.
Glycopeptide profiling
This method combines peptide sequencing and glycan identification to pinpoint exactly where glycans attach to the protein backbone. Proteins are enzymatically digested, often by trypsin, and analyzed by LC-MS/MS. This site-specific profiling reveals both macro-heterogeneity (which sites carry glycans) and micro-heterogeneity (glycan structures at each site). Identifying glycopeptides is challenging because glycans preferentially fragment during MS analysis. Advanced methods like electron-transfer dissociation or hybrid fragmentation techniques preserve glycan–peptide linkages, enabling accurate site identification and structural assignment.
Monosaccharide composition analysis
Glycans are built from basic sugar building blocks, so monosaccharide analysis provides a compositional understanding of the molar concentration of each sugar within the glycan. For this, the glycoprotein is chemically hydrolyzed with strong acids, breaking glycans into their individual sugars. For quantitation, high-performance anion-exchange chromatography with pulsed amperometric detection is used. This method rapidly assesses glycoprotein quality changes in sugar ratios. Changes during bioprocessing might indicate manufacturing variations or protein degradation. It also ensures the absence of immunogenic non-human sugars (such as Neu5Gc or α-galactose) within the biologic.
Determination of linkage position
A classical approach for linkage determination is methylation analysis. Here, free hydroxyl groups on a released glycan are fully methylated (known as permethylation), then the glycan is hydrolyzed, reduced and acetylated. The resulting partially methylated alditol acetates are analyzed by gas chromatography-mass spectrometry, and fragment patterns reveal which hydroxyls were initially involved in glycosidic bonds. Permethylation stabilizes sialic acids and enhances ionization, making low‑abundance glycans more detectable. Alternatively, tandem MS fragmentation techniques can generate characteristic cross‑ring cleavages that distinguish linkage details, such as differentiating α2‑3 from α2‑6 sialic acid attachments when analyzed under suitable collision conditions. While a powerful technique, some isomers may remain ambiguous, so nuclear magnetic resonance spectroscopy is used for definitive linkage and anomeric configuration assignments. However, larger amounts of purified glycan are required.