Availability
Five reviews [57, 66, 72, 76, 77] included eight availability interventions (Table 4) (Fig. 2).
Summary of results. “’+’: Positive effect;…
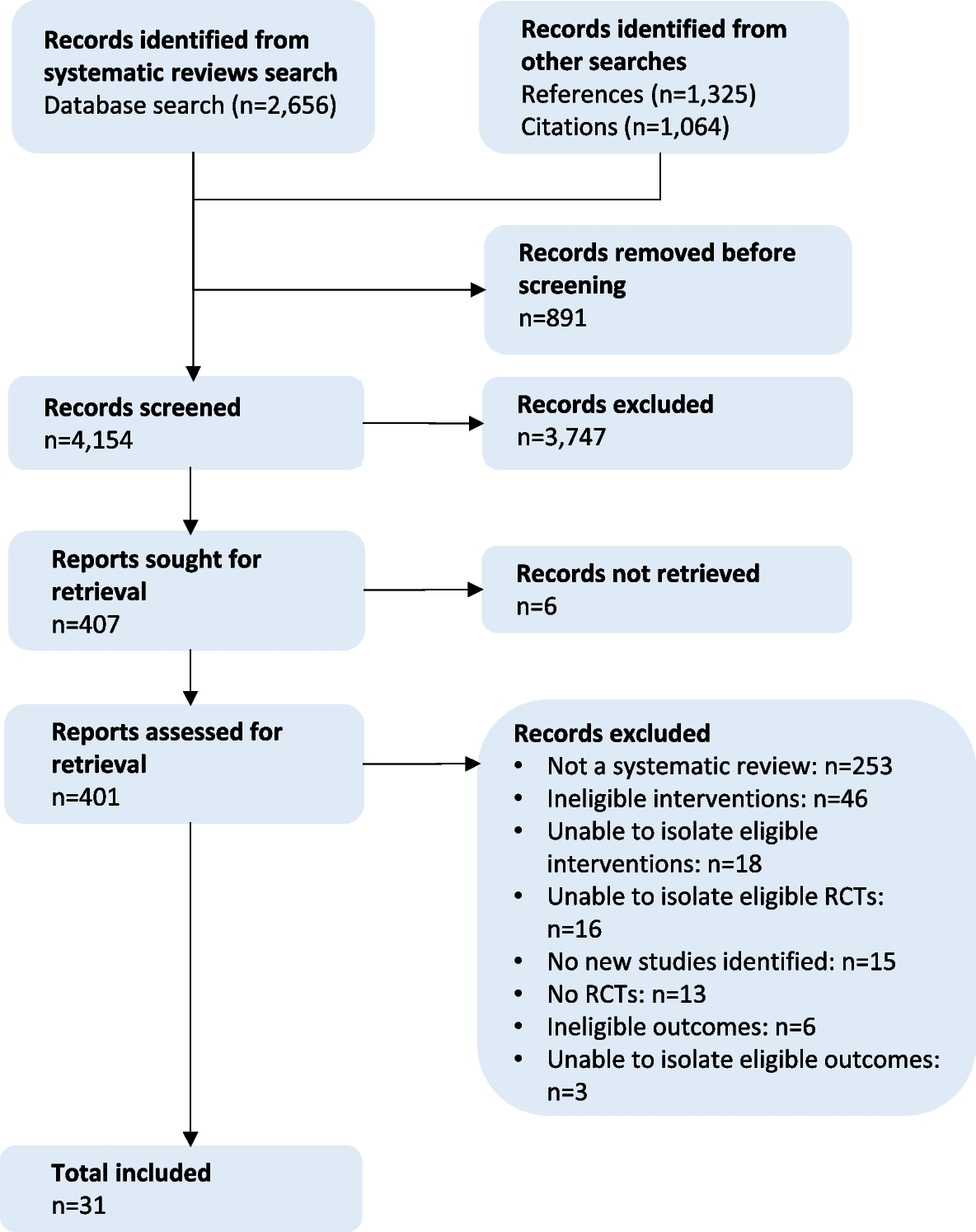
Five reviews [57, 66, 72, 76, 77] included eight availability interventions (Table 4) (Fig. 2).
Summary of results. “’+’: Positive effect;…