In 1825 Michael Faraday isolated a sweet-smelling chemical that he dubbed bi-carburet of hydrogen. That molecule, better known as benzene, is perhaps the most famous molecular ring in chemistry.
Back in 2019, for example, a team led by Simon Aldridge at the University of Oxford made an aluminum complex that inserts itself into benzene, priming it for a reaction with a tin reagent that breaks open the ring. In addition, certain enzymes can pry open the benzene ring in catechol.
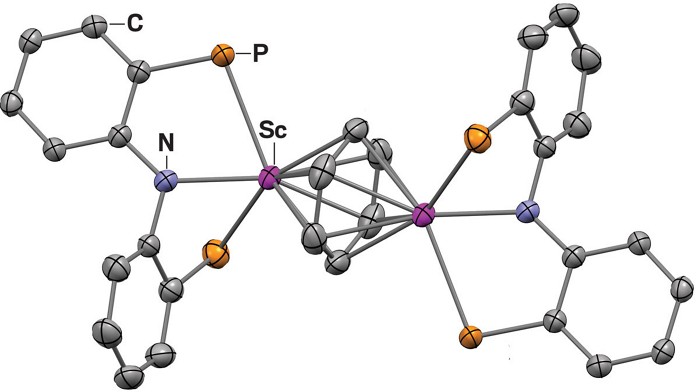
The team behind the new study instead drew inspiration from the kinds of metal complexes that can break the strong bonds in dinitrogen or carbon monoxide. First they made a scandium complex containing a pincer-like ligand that stabilizes a range of metal oxidation states. Then they mixed the complex with potassium graphite (KC8), which is a strong reducing agent, and benzene at room temperature. This formed an inverted sandwich complex in which each of benzene’s faces binds to a scandium complex. In this arrangement, the benzene carries four extra electrons in its antibonding orbitals.
While exploring the chemistry of this sandwich complex, the team found that it reacted with metal carbonyls, such as chromium hexacarbonyl, also at room temperature. Theoretical studies led by team member Xiaotai Wang of Xi’an Jiaotong-Liverpool University suggest that this involves a series of carbonyl-insertion reactions that ultimately help to lengthen and then break one of benzene’s carbon-carbon bonds.
The product is a complex containing both scandium and chromium and with a linear hexadiene unit at its heart. “We discovered this reaction by chance,” Chu says. “But after we got the crystal structure of the product, we found that it’s very beautiful.” Adding carbon monoxide gas helps the reaction along, offering yields of up to 88%, and the process works just as well with toluene.
“Scandium is always good for a surprise because it’s one of the smallest, highest-charged metal cations,” says Sjoerd Harder of FriedrichAlexander University Erlangen-Nuremberg, an organometallic chemist who uses main-group metal complexes to activate strong bonds. Harder was not involved in the new work. “It can do stuff that other metals can’t do.”
Although many different metals have previously been used to create inverted sandwich complexes of benzene, Harder doesn’t think that anyone has tried reacting them with metal carbonyls before. “It could be that the other complexes react like that, but we just don’t know it,” he says.
Chu’s team is now trying to free the organic component of the product from its metal partners and—given the relatively high cost of scandium—investigating whether other metals could act as catalysts to achieve the same ring-breaking feat. “If he’s able to do this catalytically, then we’re talking,” says Harder.