Image by Ashling Wahner & MJH Life Sciences Using AI
Domvanalimab in combination with zimberelimab and FOLFOX (leucovorin, fluorouracil, and oxaliplatin) was active with no new safety signals in patients with untreated locally advanced unresectable or metastatic gastric, gastroesophageal junction (GEJ), or esophageal adenocarcinoma, according to findings from the phase 2 EDGE-Gastric trial (NCT05329766).1 In light of these findings, investigators initiated the phase 3 STAR-221 trial (NCT05568095), which is comparing domvanalimab plus zimberelimab and chemotherapy with nivolumab (Opdivo) plus chemotherapy in the same patient population.2
“Domvanalimab is an [anti]-TIGIT monoclonal antibody,” Zev A. Wainberg, MD, a professor of medicine at UCLA and the codirector of the UCLA GI Oncology Program in Los Angeles, California, explained in an interview with OncLive®. “TIGIT is a checkpoint that is primarily on immune cells and blocking it leads to enhanced immune activation [and] also engages tumor-associated macrophages. The idea behind blocking TIGIT is that it works in concert with a PD-1 or PD-L1 inhibitor [such as] zimberelimab.”
EDGE-Gastric Provides Proof of Concept for Domvanalimab Combination
Arm A1 of EDGE-Gastric enrolled treatment-naive patients with locally advanced unresectable or metastatic gastric, GEJ, or esophageal adenocarcinoma.1 Eligible patients also needed to have measurable disease per RECIST 1.1 criteria, and an ECOG performance status of 0 or 1. Patients with HER2-positive tumors were excluded. Enrollment was permitted irrespective of PD-L1 levels.
All patients received domvanalimab at 1600 mg and zimberelimab at 480 mg; both agents were administered every 4 weeks. FOLFOX was given every 2 weeks. Treatment continued until disease progression or unacceptable toxicity.
The coprimary end points of EDGE-Gastric were overall response rate (ORR) and safety. Secondary end points included overall survival (OS), progression-free survival (PFS), disease control rate, and duration of response (DOR); these end points were evaluated in PD-L1 expression subgroups. Pharmacokinetic and biomarker data were also collected as secondary end points.
At the March 12, 2024, data cutoff, findings from EDGE-Gastric presented during the 2024 ASCO Annual Meeting demonstrated that the confirmed ORR among all patients who received the combination (n = 41) was 59% (95% CI, 42%-74%), including a complete response (CR) rate of 7%. The median DOR was 12.4 months (95% CI, 9.9-not evaluable [NE]).
Patients with a PD-L1 tumor area positivity (TAP) score of at least 5% (n = 16) achieved a confirmed ORR of 69% (95% CI, 41%-89%) with a CR rate of 6%. These respective rates were 50% (95% CI, 29%-71%) and 4% among patients with a PD-L1 TAP of less than 5% (n = 24). The median DORs among patients with a TAP of at least 5% and less than 5% were NE (95% CI, 11.5-NE) and 10.2 months (95% CI, 4.0-12.4), respectively.
The median PFS in the overall population was 12.9 months (95% CI, 9.8-13.8) and the 12-month PFS rate was 58% (95% CI, 42%-74%). The median PFS values in the patient subgroups with TAP scores of at least 5% and less than 5% were 13.8 months (95% CI, 11.3-NE) and 11.3 months (95% CI, 5.5-13.8), respectively. The respective 12-month PFS rates were 69% (95% CI, 46%-92%) and 47% (95% CI, 25%-69%).
“EDGE-Gastric is the largest dataset [of] FOLFOX plus domvanalimab and zimberelimab in [this patient population],” Wainberg said. “[Data from] the study showed a very high response rate of 59% in the overall population, [which included] patients with a PD-L1 TAP of less than 5%. The median PFS [in the overall cohort] was 12.9 months. I believe these [efficacy] signals warrant further investigation.”
In terms of safety, any-grade treatment-emergent adverse effects (TEAEs) and TEAEs related to any study drug occurred at rates of 100% and 98%, respectively. Grade 3 or higher TEAEs were reported at a rate of 73%, including 15% that were related to domvanalimab/zimberelimab and 59% that were related to FOLFOX. Notably, no serious TEAEs related to domvanalimab/zimberelimab occurred; overall, serious TEAEs were reported in 37% of patients. TEAEs resulting in study drug discontinuation, dose modifications or interruptions, or death occurred in 66%, 85%, and 2% of patients, respectively.
The most common grade 1 or 2 TEAEs included nausea (59%), fatigue (27%), diarrhea (27%), and thrombocytopenia (27%). Common grade 3 or higher TEAEs included neutropenia (51%), anemia (12%), and thrombocytopenia (7%).
In May 2025, Arcus Biosciences announced that OS data from EDGE-Gastric will be presented at a medical meeting in the fall of 2025.3
“There have been preceding data that [showed] some activity, both with FOLFOX plus domvanalimab and zimberelimab, as well as with other TIGIT antibodies in [patients with] esophageal cancers. We have seen some preliminary activity that justifies the need for a phase 3 trial,” Sam Klempner, MD, a medical oncologist at Massachusetts General Hospital and an associate professor of medicine at Harvard Medical School in Boston, said in an interview with OncLive®.
STAR-221 Aims to Confirm Benefit of Domvanalimab Plus Zimberelimab and Chemotherapy
STAR-221 is an open-label, multicenter study that is examining domvanalimab and zimberelimab plus chemotherapy in approximately 1040 adult patients with treatment-naive locally advanced unresectable or metastatic gastric, GEJ, or esophageal adenocarcinoma (Figure).2,4 Eligible patients must also have an ECOG performance status of 0 or 1 and at least 1 measurable lesion per RECIST 1.1 criteria. Those with HER2-positive disease, clinically significant cardiovascular disease, untreated, symptomatic, or actively progressing central nervous system metastases, or disease progression within 6 months of completion of neoadjuvant or adjuvant therapy are excluded from the study.
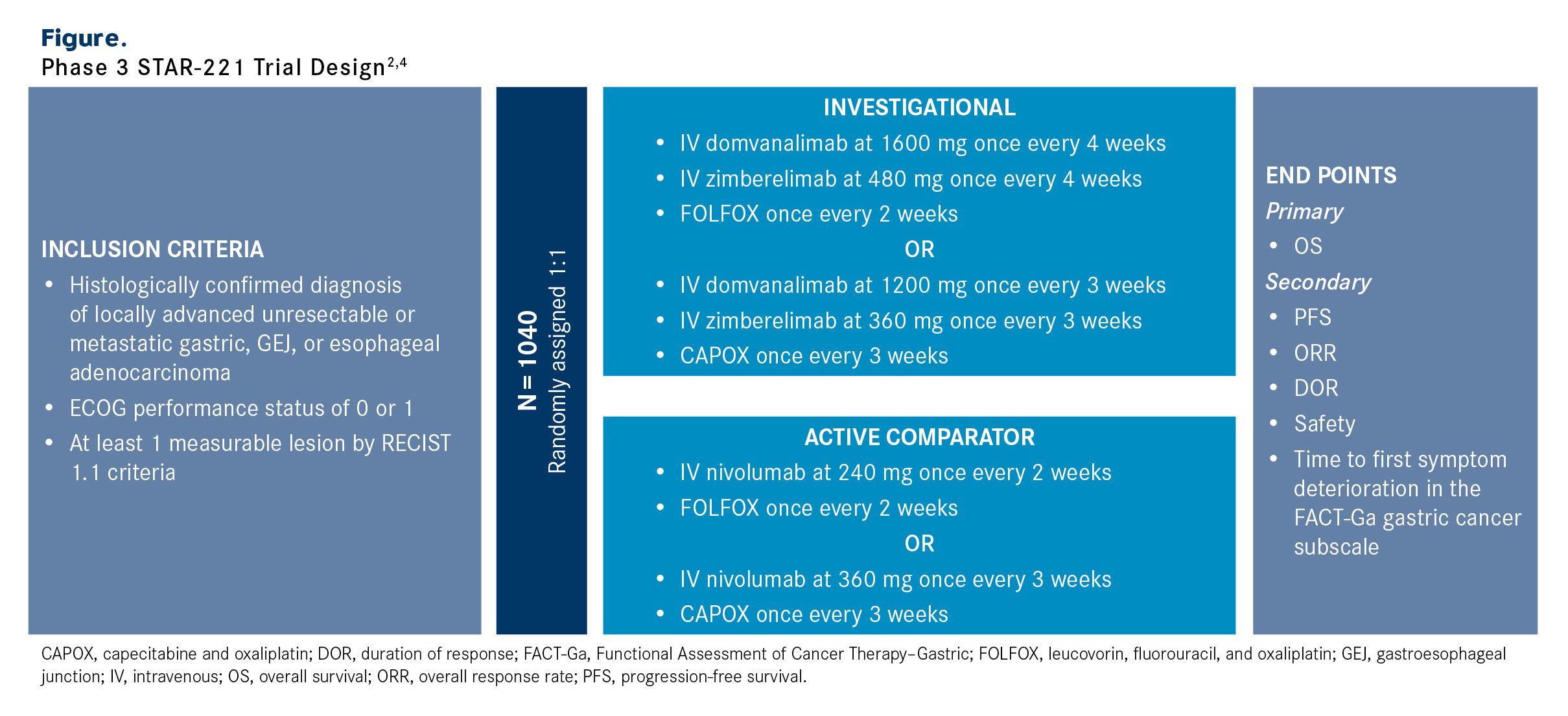
Patients will be randomly assigned 1:1 to the investigational or active comparator arm. In the investigational arm, patients will receive domvanalimab at 1600 mg and zimberelimab at 480 mg once every 4 weeks in combination with FOLFOX every 2 weeks or domvanalimab at 1200 mg and zimberelimab at 360 mg plus CAPOX (capecitabine and oxaliplatin) every 3 weeks. In the active comparator arm, patients will receive nivolumab at 240 mg and FOLFOX every 2 weeks or nivolumab at 360 mg and CAPOX every 3 weeks.
“The comparator arm is an active combination, and it’s probably our best standard for this patient population,” Klempner commented. “I don’t believe there is a more active control arm that could have been [selected], so that was not a barrier to accrual. The trial is fully accrued, and we’re awaiting the readout.”
The primary end point is OS. Secondary end points include PFS, ORR, DOR, safety, and time to first symptom deterioration according to the Functional Assessment of Cancer Therapy–Gastric subscale.
The estimated primary completion date is December 2026. In May 2025, Arcus Biosciences announced that the first data readout from STAR-221 is expected in 2026.3
“If [STAR-221] meets its primary end point and shows a statistically significant and clinically meaningful improvement in OS, I believe [this regimen] would become the de facto standard of care in patients with newly diagnosed disease,” Wainberg said. “However, there will be some nuance in [terms of] which patients [are best suited for this regimen]. We acknowledge that checkpoint inhibitors should be used primarily in patients with a PD-L1 expression of greater than one. It will be important for us not just to prove that the study met its primary end point in the intent-to-treat [population], but also in the cohorts in which checkpoint inhibitors are the standard [of care].”
References
- Janjigian YY, Oh DY, Pelster M, et al. EDGE-Gastric arm A1: phase 2 study of domvanalimab, zimberelimab, and FOLFOX in first-line (1L) advanced gastroesophageal cancer. Presented at: 2024 ASCO Annual Meeting; May 31 to June 4, 2024; Chicago, Illinois. Abstract 433248.
- A clinical trial of a new combination treatment, domvanalimab and zimberelimab, plus chemotherapy, for people with an upper gastrointestinal tract cancer that cannot be removed with surgery that has spread to other parts of the body (STAR-221). ClinicalTrials.gov. Updated June 26, 2025. Accessed July 15, 2025. https://clinicaltrials.gov/study/NCT05568095
- Arcus Biosciences reports first-quarter 2025 financial results and provides a pipeline update. News release. Arcus Biosciences. May 6, 2025. Accessed July 15, 2025. https://investors.arcusbio.com/investors-and-media/press-releases/press-release-details/2025/Arcus-Biosciences-Reports-First-Quarter-2025-Financial-Results-and-Provides-a-Pipeline-Update/default.aspx
- Klempner SJ, Shitara K, Sison A, et al. STAR-221: a randomized, open-label, multicenter, phase 3 trial of domvanalimab, zimberelimab, and chemotherapy versus nivolumab and chemotherapy in previously untreated, locally advanced, unresectable or metastatic gastric, gastroesophageal junction, and esophageal adenocarcinoma. J Clin Oncol. 2023;41(suppl 16):TPS4206. doi:10.1200/JCO.2023.41.16_suppl.TPS4206