Study design
This multi-national population-based cohort study presents routinely collected data from Danish, English, and Swedish patient registries and electronic health records that were linked to national statistics in each country.
Data sources
We extracted individual participant data from the Danish National Patient Registry (DNPR), Hospital Episode Statistics Admitted Patient Care database (HES APC) for England, and from the Swedish National Patient Register (SNPR) [12,13,14]. For simplicity, the term registry will be used in the following for all three countries.
The DNPR includes data from all Danish hospitals, both public and private, covering inpatient care and, since 1995, also including outpatient care. It captures information such as diagnoses, surgical procedures, treatments, complications, and hospital admissions. The DNPR also records information on outpatient visits to specialist clinics and emergency department visits [12]. After identifying the Danish study population in the DNPR, it was linked to the Civil Registration System (CRS) by the unique 10-digit personal identification number given to all Danes at birth or immigration since 1968. This allows individual-level linkage of data between multiple registries. The CRS also contains information on date of birth, age, sex, and vital status [17].
HES APC is a dataset containing data on all remunerated activity within National Health Service hospitals (NHS) or NHS-funded care in England where the patient requires an inpatient stay in secondary care. This includes day-case procedures and provides data on primary and secondary clinical diagnoses. Data can be linked at a patient level to all other secondary care episodes within the NHS, in addition to national mortality data [14].
The SNPR contains information from inpatient care and, since 2001, also from outpatient care, thus including hospital admissions, emergency department visits, and specialist outpatient visits. It covers data from all Swedish hospitals, both public and private, and includes information on patient demographics, diagnoses, treatments, and procedures [13]. To ensure a high degree of completeness from the SNPR, the Swedish data extract did not include data from before 2001.
Information about the specific content of each national patient registry is provided in Table S1 (Additional file 1: Table S1) [12,13,14, 17,18,19,20,21]. Furthermore, population data for incidence calculation were extracted from national statistics in each country [18, 20, 21]. All individual-level data were provided in a de-identified format.
Participants
Based on the International Classification of Diseases, 10th Revision (ICD-10), all adults (≥ 18 years) with PHF (S.42.2*) were identified in the three national patient datasets [11,12,13].
The participants’ first fracture on each arm was included. In cases where laterality codes were missing, only the first fracture in one arm was included. Each index episode was analysed as an independent observation.
The exclusion criteria included the presence of bilateral PHF, any concurrent injury, and cancer registered at the same episode/index date as the fracture. This was to exclude polytrauma and pathological fractures. Specific details of the ICD-10 codes used for including and excluding patients, as well as recording comorbidities and SAEs, are found in Table S2 (Additional file 1: Table S2-S4).
Primary outcome
The primary outcome variables were the numbers of pre-defined surgical procedures for PHF. The Nordic Medico-Statistical Committee (NOMESCO) Classification codes (NCSP-codes) were used to identify surgical procedures linked to the ICD-10 code in Denmark and Sweden, while Operational Procedure Codes, 4th Edition (OPCS-4) were used to identify surgical procedures linked to the ICD-10 code in England [19, 22]. The predefined surgical procedures were: plate fixation, screw fixation, K-wire fixation, intramedullary nail (IM nail) fixation, external fixation, and arthroplasty. If patients had surgery within 30 days after the fracture index date, surgery was considered as initial treatment. If the index date of the surgical procedure was later than 30 days after the fracture, the initial treatment was categorised as non-operative, thus, they were excluded from the analysis. A complete coding list for the surgical procedures can be found in Table S2 (Additional file 1: Table S2). If more than one surgical procedure was performed on the same date and no data were available to classify which was the primary, the procedure was classified using a predefined hierarchy (Additional file 1: Table S5).
Secondary outcomes
Each specific surgical procedure was linked to the first episode of each SAE in a set of SAEs, occurring within 30 days after the index date of the surgical procedure. The SAEs were identified based on ICD-10 codes and included: stroke, respiratory tract infection, myocardial infarction, pulmonary embolus, urinary tract infection, and acute renal failure. In addition, mortality within 30 and 90 days was counted.
Covariates
To identify potential confounding, age, sex, and comorbidities were compared between surgical subgroups and between countries. Information on history of ischaemic heart disease (IHD), diabetes mellitus (DM), and chronic obstructive pulmonary disease (COPD) was extracted. The overall level of comorbidity was calculated as the CCI using the algorithm by Quan et al. [23]. In Denmark and Sweden, a one-year lookback period was applied when extracting past medical history and CCI variables, whereas in England, no time limit was applied to the lookback period due to the limitation of no outpatient and emergency department information within the English dataset.
Data processing
The primary investigators, responsible for the data in each participating country, cleaned and processed their national data. Data management flow charts for each country can be found in supplementary material (Additional file 2: Fig. S1a,b,c). Patient-level analyses were performed securely according to locally agreed data management procedures. Do-files were developed and shared among the three countries to enable reproducible analytical pipelines in each centre.
Statistical analysis
National baseline characteristics of patients divided by surgical subtype after isolated PHF were presented to identify potential differences in population profiles and sources of bias.
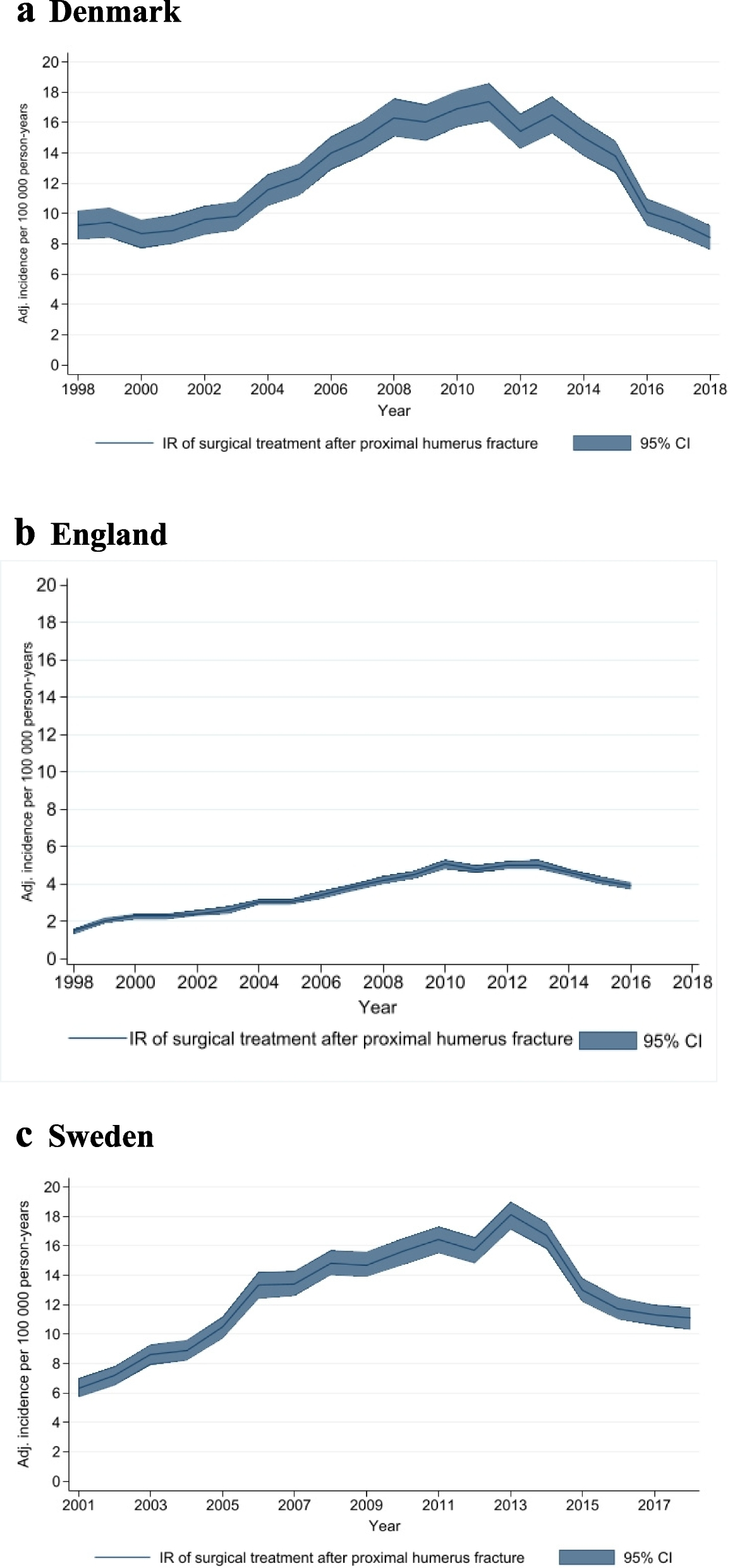
For each country, incidence rates (IR) per 100,000 person-years with 95% confidence intervals (95% CI) were calculated for all surgically managed PHFs and for each surgical subtype. This was done by using national population estimates. In addition, age- and sex-specific IRs were calculated. The annual surgical IRs for all three countries were plotted against the date of a Cochrane systematic review and a large clinical trial, to determine if there was an impact of trial recruitment or publication of high-quality evidence [9, 10].
SAEs, occurring within 30 days of each surgical procedure, were presented as cumulative incidence proportions (hereafter referred to as incidence) with 95% CI, assuming a normal approximation to the Poisson distribution for random count data.
Survival analysis using a Kaplan–Meier method was undertaken to show survival over the first post-operative year, as well as for the entire study period. Patients were censored at the date of death or end of follow-up, whichever came first.
Multivariable logistic regression analysis was undertaken to determine the impact of age (in 20-year age bands), sex, and overall comorbidity (using CCI categorised as (0–1), (2–3), (4 +)) upon the rise of SAEs within 30 days as well as 30-day, 90-day, and 1-year post-operative mortality.
Aggregated results from each country were compiled by the first author. The statistical package Stata (version 17, StataCorp, College Station, TX) was used for data cleaning, pre-processing, and statistical analyses.
The study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [24].