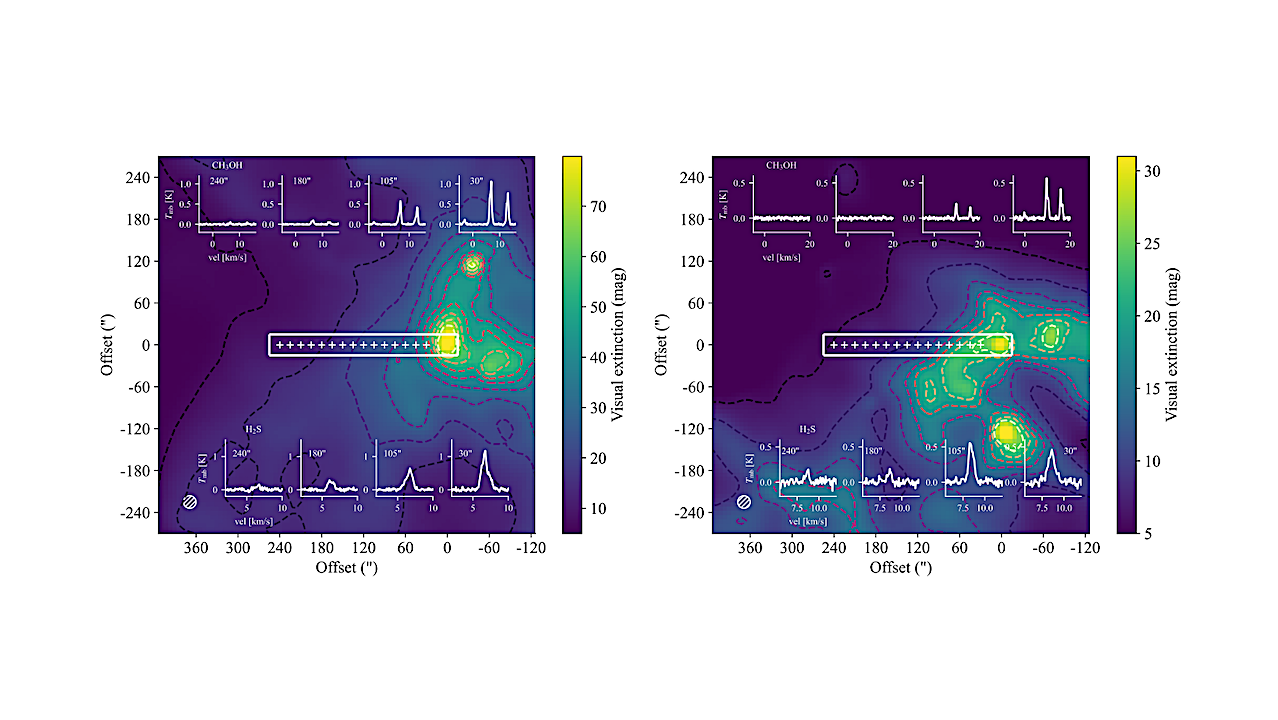
Visual extinction maps of the observed regions. Contours in the Barnard 1b map (left) correspond to [7, 14, 21, 28, 35, 42, 49, 56, 63, 70] mag levels. The contours in the IC 348 map (right) correspond to [6, 9, 12, 15, 18, 21, 24] mag levels. These maps were obtained from the τ850 optical depth maps in Zari et al. (2016) applying the K-band to V-band extinction ratio of ∼ 1/16 (Nishiyama et al. 2008). The crosses mark the pointings from where data was taken. Selected spectra of CH3OH lines at 90 GHz (Table 2) and H2S 11,0 → 10,1 line observed at different offsets are included in the maps. — astro-ph.GA
Grain-surface chemistry plays a crucial role in the formation of molecules of astrobiological interest, including H2S and complex organic molecules (COMs).
They are commonly observed in the gas phase toward star-forming regions, but their detection in ices remains limited. Combining gas-phase observations with chemical modeling is therefore essential for advancing our understanding of their chemistry.
In this paper we investigate the factors that promote or hinder molecular complexity combining gas-phase observations of CH3OH, H2S, OCS, N2H+, and C18O with chemical modeling in two dense cores: Barnard-1b and IC348. We observed millimeter emission lines of CH3OH, H2S, OCS, N2H+, and C18O along strips using the IRAM 30m and Yebes 40m telescopes. We used the gas-grain chemical model Nautilus to reproduce the observed abundance profiles adjusting parameters such as initial sulfur abundances and binding energies.
H2S, N2H+ and C18O gas-phase abundances vary up to one order of magnitude towards the extinction peak. CH3OH abundance remains quite uniform. These abundances can only be reproduced assuming a decreasing sulfur budget, which lowers H2S and enhances CH3OH abundances. Decreasing binding energies, which are expected in CO-rich apolar ices, are also required.
The sulfur depletion required by H2S is generally higher than that required by CH3OH, suggesting unknown sulfur sinks. These findings highlight the intricate relationship between sulfur chemistry and COM formation, driven by the competition between sulfur and CO for hydrogen atoms.
Our study emphasizes that the growth of CO ice and the progressive sequestration of hydrogen atoms by sulfur are critical in determining whether chemical complexity can develop, providing key insights into the early stages of star and planet formation.
D. Navarro-Almaida, A. Taillard, A. Fuente, P. Caselli, R. Martín-Doménech, J. J. Miranzo-Pastor
Comments: 46 pages and 37 figures. Accepted for publication in Astronomy and Astrophysics
Subjects: Astrophysics of Galaxies (astro-ph.GA)
Cite as: arXiv:2507.17595 [astro-ph.GA] (or arXiv:2507.17595v1 [astro-ph.GA] for this version)
https://doi.org/10.48550/arXiv.2507.17595
Focus to learn more Submission history From: David Navarro-Almaida
[v1] Wed, 23 Jul 2025 15:27:16 UTC (2,364 KB)
https://arxiv.org/abs/2507.17595
Astrobiology, Astrochemistry,