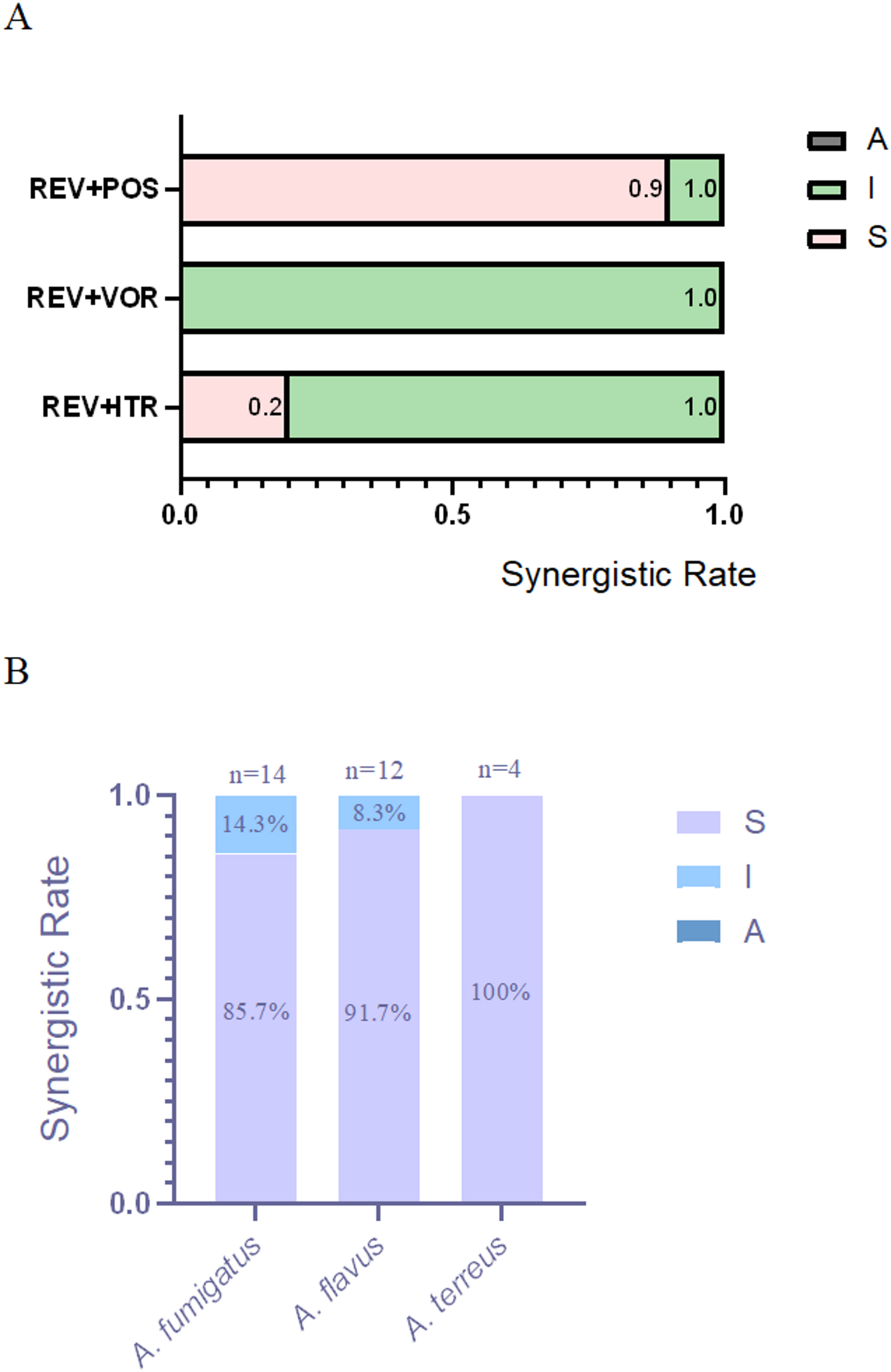
In vitro antifungal activities of the tested drugs
The MIC values of the tested drugs used to treat the Aspergillus isolates were ≥ 16 µg/mL for REV, 0.5–4 µg/mL for ITR, 0.5–1 µg/mL for VOR, and 0.5–1 µg/mL for POS (Table 1).
In vitro interactions of REV and the antifungal agents
When REV and ITR were combined, the MIC values of these two drugs against the clinical isolates decreased to 0.25–8 µg/mL and 0.25–4 µg/mL, The drug combination exhibited synergistic effects (FICI ≤ 0.5) against 20% of the evaluated Aspergillus isolates, comprising 2 A. fumigatus strains, 1 A. flavus strains, and 3 A. terreus strains (Tables 1 and 2).
When REV and POS were combined, the MIC values of these two agents decreased to 0.25–8 µg/mL and 0.0625–1 µg/mL, The drug combination exhibited synergistic effects (FICI ≤ 0.5) against 90% of the evaluated evaluated Aspergillus strains, comprising 12 A. fumigatus strains, 11 A. flavus strains, and 4 A. terreus strains (Tables 1 and 2).
When REV and VOR were combined, the MIC values of these two antifungal agents decreased to 0.25 µg/mL and 0.125–1 µg/mL, respectively, and no synergistic or antagonistic effects on Aspergillus isolates were observed (Tables 1 and 2).
In the REV + POS alliance,A. fumigatus: 85.7% isolates (12/14) showed synergy;A. flavus: 91.7% isolates (11/12) showed synergy༛A. terreus: 100% isolates (4/4) showed synergy. POS consistently enhanced REV activity across all species, suggesting broad-spectrum potential.
In the REV + ITR alliance, A. terreus: 75% synergy (3/4 isolates), the highest among species.;A. fumigatus: 14.3% synergy (2/14 isolates)༛A. flavus: 8.3% synergy (1/12 isolates).ITR may have niche utility against A. terreus but limited synergy with REV for other species.
In the REV + VOR alliance, No synergy observed in any isolate (0/30 total); VOR is not a viable partner for REV in these Aspergillus species.
Thus, we conclude that: REV + POS was universally effective (85.7–100% synergy), but most potent against A. flavus (91.7%);REV + VOR failed in all cases, while REV + ITR showed sporadic activity (useful only for A. terreus).The synergy data is now explicitly compared by species, demonstrating that REV + POS is the most promising combination, particularly for A. flavus. The lack of synergy with VOR is also highlighted as a critical negative result.(Fig. 1).
The Synergistic Rate of REV Combined with Azoles against Aspergillus spp. Note: A, interaction profile of REV combined with ITR, VOR and POS against Aspergillus spp; B, REV + POS interaction profiles across Aspergillus species
Construction of knockout strains for the genes encoding MFS transporters in A. fumigatus
Twelve MFS transporter genes were selected by NCBI database, and 12 MFS knockout strains were successfully constructed by PCR validation and sequencing analysis. The knockdown construction process is shown in Fig 2. Electrophoresis showed that the relevant 12 target genes failed to amplify the bands, while the pyrG gene amplified clear bands, and the electrophoretic profiles of 10 target genes is shown in Fig 3. Target genes AF-MFS32 and AF-MFS35 are shown in Fig 4B; moreover, confirming successful knockout of AF-MFS32 and AF-MFS35, electrophoresis analysis revealed 1.6 kb fragments amplified using AF-MFS32 downstream primers P4 and Awm-F1 and 1.8 kb fragments using AF-MFS35 upstream primers P1 and Carslan-R4 in Fig 4A.
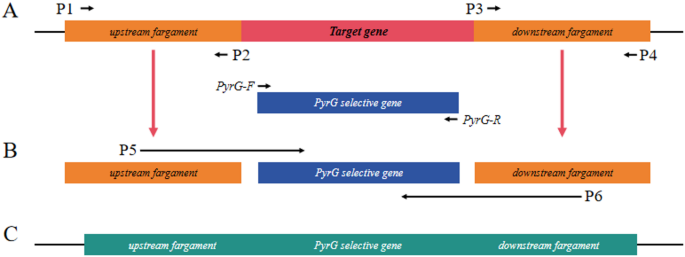
Schematic diagrams of primer binding sites and gene tissues for gene knockout and knockout verification. A Amplify the upstream and downstream DNA fragments of target geneand amplify the pyrG fragments labeled for screening. B Construct the fusion PCR products (UP, pyrG, DOWN). C Construct knockout confirmatory products to detect whether the gene has been successfully knocked out
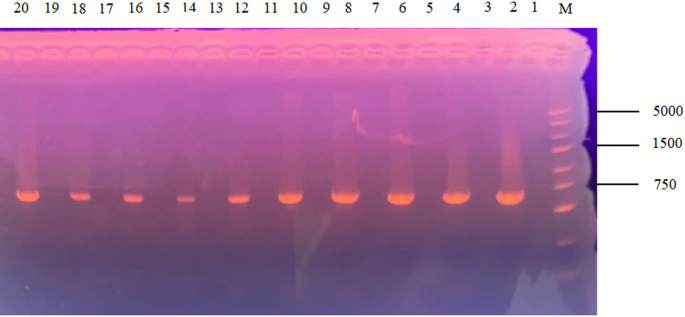
Electrophoretic gel for mutant confirmation. Note: M: DNA5000 Marker; 1, 3, 5, 7, 9, 11, 13, 15, 17, 19: No obvious bands were observed in the amplification of the target gene; 2, 4, 6, 8, 10, 12, 14, 16, 18, 20: Ten of these target genes corresponding to the pyrG fragment
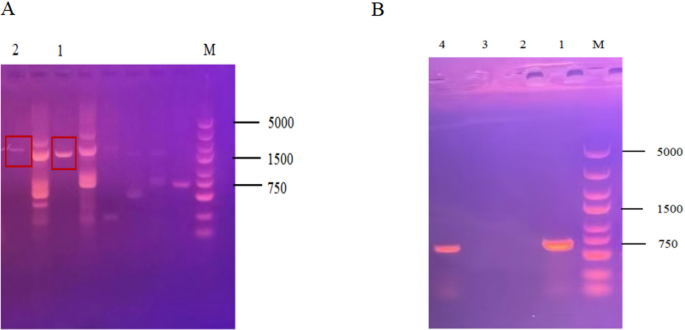
Verified electrophoretic gel diagram. Note: A: M: DNA5000 Marker; 1: The bands were amplified with primers P4 and Awm-F1 downstream of AF-MFS32; 2: The bands were amplified with primers P1 and Carslan-R4 upstream of AF-MFS35. B:1: The AF-MFS32 pyrG fragment; 2: No obvious bands were observed in the amplification of the target gene; 3: No obvious bands were observed in the amplification of the target gene; 4: The AF-MFS35 pyrG fragment
In vitro interaction of REV with antifungal agents in MFS transporter genes knockout strains
When REV, ITR, and VOR were combined, none of the 12 knockout strains of MFS transporter gene showed obvious synergistic activity. When REV and POS were combined, ten of these isolates demonstrated a synergistic activity. However, in the two knockout strains of ΔAF-MFS32 and ΔAF-MFS35, the synergistic effect of REV and POS was reversed. (Table 3)
Disk diffusion method
The combination of REV and POS exerted a significantly lower inhibitory effect on the ΔAF-MFS32 and ΔAF-MFS35 than on the control WT suggesting a statistically significant difference (P < 0. 05). (Fig 5)
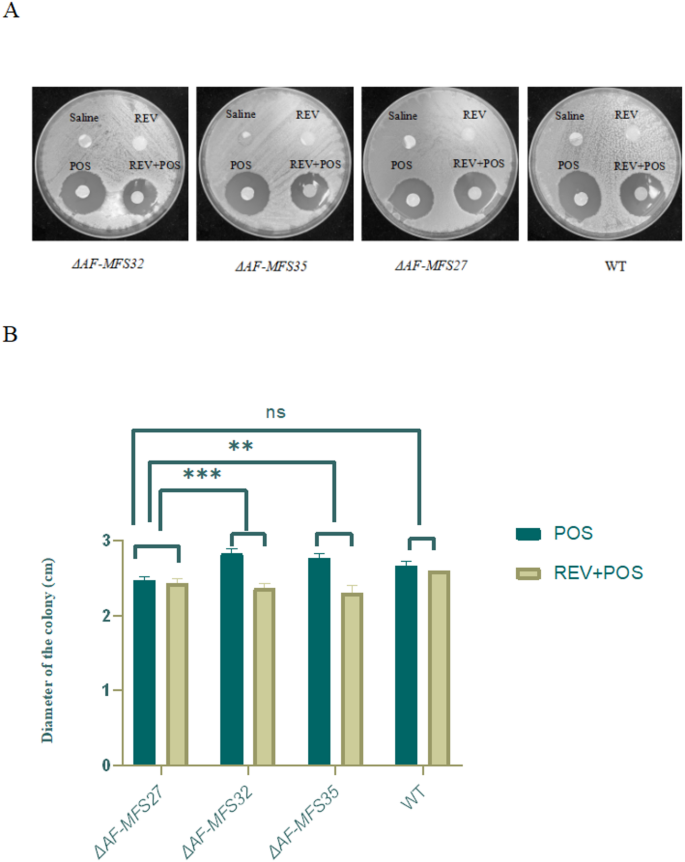
Results of disk diffusion method. Note: A When REV combined with POS, the inhibition zone of ΔAF-MFS32, ΔAF-MFS35 was significantly smaller than that of control group ΔAF-MFS27. B REV: Revaprazan (8 µg/ml);POS: posaconazole (8 µg/ml). **P < 0.01,***P < 0.005
Rhodamine 6G – Efflux pump activity assay
The experiment to assess the efflux pump activity of knockout strains ΔAF-MFS32, ΔAF-MFS35, and the wild-type strain WT uses Rhodamine 6G as a substrate for the efflux pump. The accumulation and efflux of Rhodamine 6G reflect the pump’s function. According to the time-fluorescence curve, the efflux pump activity of the wild-type strain WT is significantly enhanced when REV and POS are used together compared to when POS is used alone, with a statistically significant difference. In contrast, the differences in efflux pump activity between the knockout strains ΔAF-MFS32 and ΔAF-MFS35 when POS is used alone and when REV and POS are used together are not statistically significant (Fig. 6).
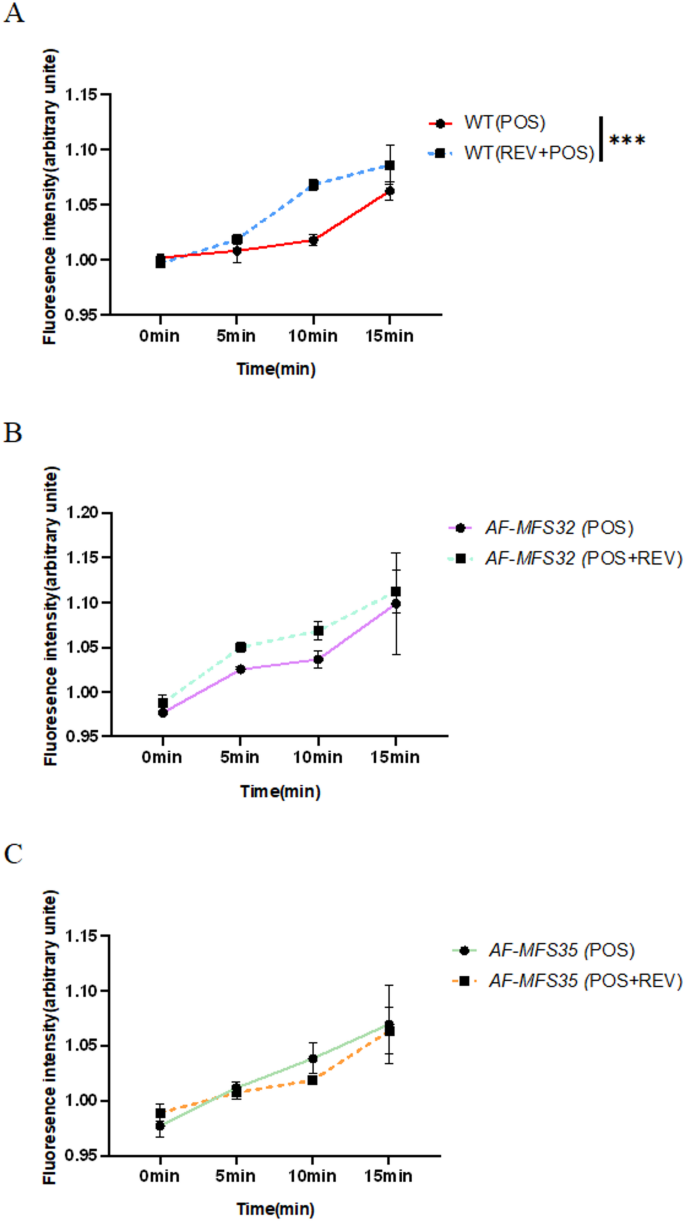
Rhodamine 6G efflux pump activity. Note: A: When the wild-type strain WT was used together with POS in REV and POS, the efflux pump activity was significantly enhanced compared with that of POS alone, which was statistically significant. B No statistically significant difference in efflux pump activity was observed in the AF-MFS32 knockout strain between POS monotherapy and its combination with REV and POS. C No statistically significant difference in efflux pump activity was observed in the AF-MFS35 knockout strain between POS monotherapy and its combination with REV and POS
Pharmacological dissection of Rhodamine 6G efflux in ΔAF-MFS32 and ΔAF-MFS35 knockout strains
To unambiguously distinguish the types of transporters mediating Rh6G efflux, we further employed pharmacological inhibitors for validation. The experimental results (Fig. 7) demonstrated that in the WT strain, treatment with the proton ionophore CCCP (which abolishes the function of MFS transporters) resulted in a significant suppression of Rh6G efflux activity compared to the DMSO control group (p < 0.01). Subsequent addition of the ABC transporter inhibitor sodium vanadate led to a further significant inhibition of efflux activity (p < 0.05). This confirms that in the wild-type strain, Rh6G efflux is cooperatively mediated by MFS transporters (major contribution) and ABC transporters (minor contribution).
In contrast, both knockout strains, ΔAF-MFS32 and ΔAF-MFS35, exhibited patterns strikingly different from that of the WT. No statistically significant differences were observed between the REV + POS combination treatments in either knockout (p > 0.05). More importantly, CCCP treatment failed to effectively inhibit the efflux activity in both knockout strains (p > 0.05 compared to their respective DMSO controls), and no significant difference was observed between the CCCP and CCCP + Vanadate treatments.
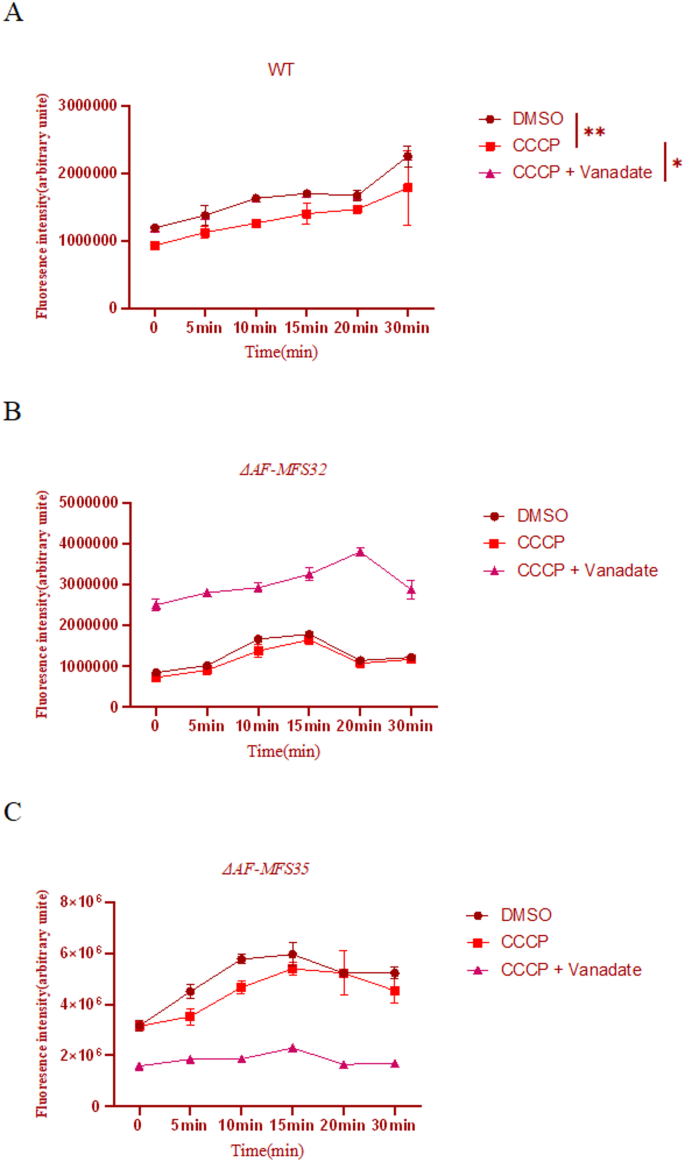
Pharmacological dissection of Rhodamine 6G efflux in ΔAF-MFS32 and ΔAF-MFS35 knockout strains. Note: (A–C) Time-course analysis of intracellular Rhodamine 6G (Rh6G) accumulation in thewild-type (WT, A), ΔAF-MFS32 (B), and ΔAF-MFS35 (C) strains under different pharmacological treatments. Fluorescence intensity is inversely proportional to efflux pump activity. Treatments: DMSO (vehicle control), CCCP (20 µM, protonophore that collapses the proton motive force and inhibits MFS transporters), and CCCP + Vanadate (50 µM, ABC transporter inhibitor). Data are presented as mean ± SEM (n = 3).**P < 0.01;*P < 0.05
In the WT strain (A), CCCP treatment caused a dramatic increase in Rh6G accumulation (inhibition of efflux), indicating that efflux is primarily dependent on the proton gradient and thus mediated by MFS transporters. Further addition of vanadate significantly increased accumulation, revealing a minor contribution from ABC transporters.The ΔAF-MFS32 mutant (B) exhibited a altered response: CCCP treatment failed to significantly inhibit efflux, and no additional effect was observed with vanadate, suggesting a shift to a non-MFS, non-ABC efflux mechanism.The ΔAF-MFS35 mutant (C) showed complete loss of efflux function, as indicated by consistently high Rh6G accumulation across all treatments, identifying AF-MFS35 as an essential component of the efflux pump.