A MAJOR new study has revealed that the COVID-19 infection in children poses a far greater risk of heart and inflammatory complications than the vaccination. Researchers found that heart conditions lasted longer and occurred more frequently…
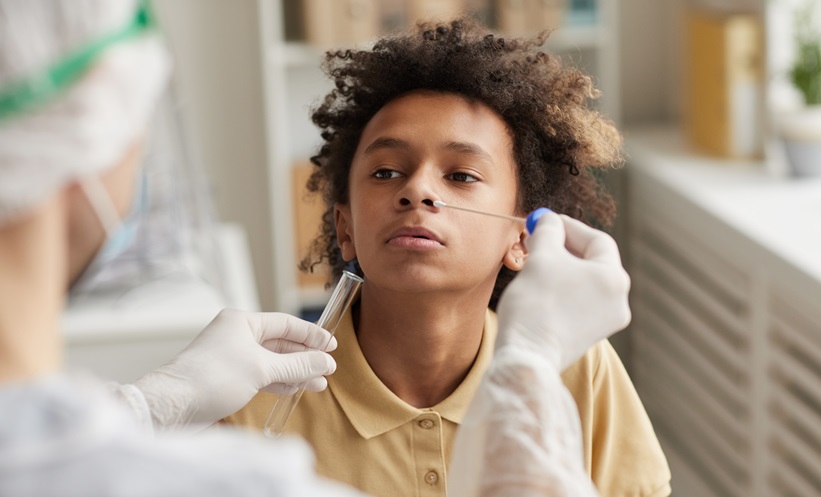
A MAJOR new study has revealed that the COVID-19 infection in children poses a far greater risk of heart and inflammatory complications than the vaccination. Researchers found that heart conditions lasted longer and occurred more frequently…