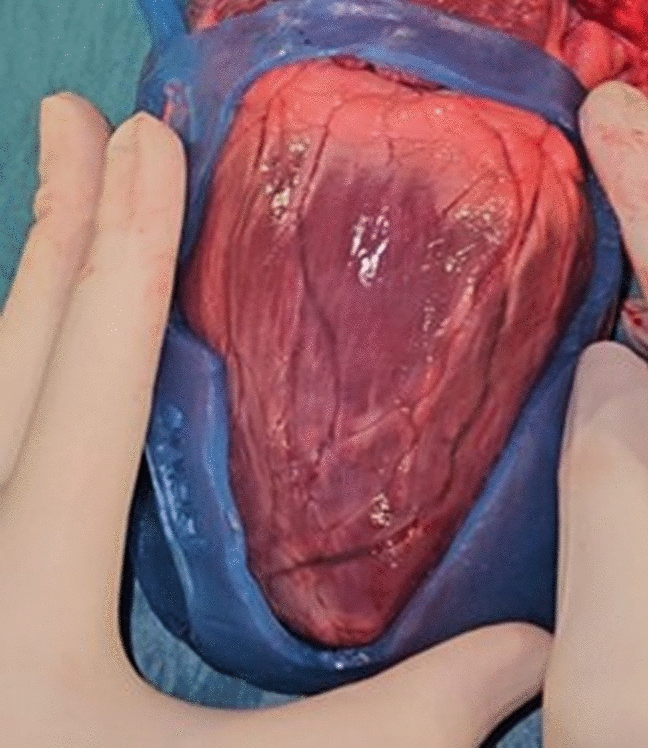
This preliminary study is the first to assess the use of HCR wrapping for patient-specific guides in VT scar ablation.
Innovative solutions are needed to precisely identify and target complex epicardial substrate during concomitant open-chest surgery—enabling accurate localization on the arrested heart—and in stand-alone procedures, where prolonged surgery and the need for an EP team in the room present additional challenges. Epicardial guides would allow the surgeon to improve navigation, accuracy, safety, and therapeutic efficacy in difficult-to-treat ablation patients.
Herein, we aimed to develop a feasible, cost-effective, and rapid method for creating epicardial surgical guides, focusing on early feasibility and technical viability. Compared to traditional 3D printing methods, HCR molding offers an efficient and affordable alternative that yields promising potential.
Rationale behind silicone and molding
The emergence of 3D printing technologies in the last decade has created abundant application opportunities in the field of precision medicine and surgical education [10]. However, 3D printing resins face challenges, such as brittleness, poor dimensional accuracy and low mechanical strength, especially after post-processing and sterilization, as well as limited material options, high costs, and lack of standardization [11,12,13]. In addition, uncured monomers or leachates may release cytotoxic byproducts, further complicating their use in surgical environments [14]. To overcome the limitations of traditional 3D printing resins, we chose a silicone-based solution—an elastomer widely used in the medical field [15]. Some silicones are approved for long-term implantation and have a proven track record in dynamic (cardiovascular) surgical environments. With its flexibility, durability, stability, versatility, and resistance to extreme temperatures, silicone can be fully customized to meet the specific needs of this epicardial application.
Although silicone modeling has been used to create customized molds [16,17,18], no studies have fabricated silicone specifically as an epicardial guide, posing unique challenges in identifying a material that meets all necessary criteria. Material choice is a critical factor to the success of a patient-specific medical device. Properties such as silicone hardness and flexibility help the guide maintain shape and function despite the heart’s constant motion, which prevents gaps that could lead to uneven lesions.
We also considered liquid silicone rubber molding, a widely used technique in medical device manufacturing and studies. However, this method requires creating an inverted mold with several components (multi-part mold system, vent channels, and silicone channels) that must be precisely aligned and securely connected using interlocks to prevent movement. This makes the process significantly more labor-intensive and less suitable for a streamlined, efficient, and rapid customized workflow for ablation treatments. Another barrier is that the liquid silicone for our required durometer would be too viscous at room temperature, making injection into the mold difficult and prone to air entrapment. We also considered direct 3D silicone printing, a more novel approach. However, it was not our preferred choice due to potential challenges with support structures, layer adhesion, and viscosity control, which could compromise integrity and make the process tedious.
An advantage of silicone wrapping is that sculpting results in a smoother inside surface due to the natural smoothness of milled silicone; unlike 3D printing, which can create rougher surfaces. This technique also allows customization using different silicones, curing times, and pigments to meet tailored requirements.
Thermal insulation and procedural safety
The guide manufacturer is responsible for ensuring compliance with design requirements (form, fit, and function) throughout the entire device’s life cycle [19]. A critical consideration in the development of surgical guides is their thermal insulation performance under ablative stress. These properties are vital for two primary reasons: ensuring procedural safety during targeted ablation and protecting adjacent, non-target anatomy from thermal injury. The material’s electrical non-conductivity reduces the risk of unintended heating when ablation instruments come into accidental contact with the guide’s edges—a scenario that can result in localized temperatures as high as 70–90 °C during radiofrequency ablation or below 0 °C during cryo-ablation, potentially causing collateral tissue damage. Due to the lack of conductivity data in the material datasheet, preliminary tests were conducted simulating accidental instrument-guide contact. These experiments confirmed that the guide effectively prevented thermal injury to underlying tissue. However, further comprehensive thermal and mechanical characterization—particularly post-processing and sterilization—remains necessary to validate the material’s suitability for clinical application.
Design considerations
Guide stability on the epicardial surface is of critical importance to ensure precise lesion placement. Material testing indicated that silicone with a 70 Shore A hardness and 2–3 mm thickness provided an optimal balance between flexibility and intraoperative stability on the beating heart. In addition, preliminary foldability tests were conducted to assess suitability for minimally invasive approaches, revealing that the current guide designs are too bulky for efficient trocar introduction, largely due to excess material at the apex base. However, this limitation is expected to be less significant in smaller, target-specific guide designs. Future work should explore alternative silicones and ensure uniform thickness without excess material. Moreover, to facilitate insertion and removal of the guide through a trocar, incorporating a secure locking system with small tabs on the lateral sides that click when folded might prove useful.
Finally, an important challenge in epicardial guide use for minimally invasive stand-alone ablation is achieving stable anchoring of the model on a beating heart. Potential solutions we considered include micro-suction ports, adhesive surfaces, or specialized gripping materials.
Broader clinical applicability and translational pathway
While the guide in this feasibility study was designed to cover a broad scar region, the end objective is to use preprocedural input from the EP to incorporate predefined ablation targets in form of ‘windows’ in the mask, directly providing the surgeon with an ablation roadmap or lesion set. By providing intra-operative guidance, it could enhance precision and reduce reliance on full real-time mapping and a complete EP team, since lesions are identified pre-operatively. However, the customization of these ablation openings requires further finetuning as they depend on patient pathology, surgical approach (open-chest or minimally invasive), and the type and size of the catheter used.
A crucial early step in clinical translation is assessing biocompatibility and characterizing the sterilized silicone material to confirm its suitability, thermal stability, mechanical integrity, and overall safety for clinical use. Preclinical validation is essential to rule out risks, such as loose fragments or allergic reactions, and to assess the guide’s fit, stability, efficacy and precise electro-anatomic alignment. Swine models are commonly used due to physiological similarity to humans and comparable heart rates, beginning with open-chest studies and advancing to minimally invasive approaches [20]. For these experiments, subject-specific guides are fabricated from pre-operative cardiac CT. While device alignment partially relies on visible anatomical landmarks (aorta, pulmonary artery ring, and left ventricular apex)—which are accessible via both open-chest and thoracoscopic approaches—epicardial electro-anatomic mapping is still needed during early translational phases. Alignment accuracy can be quantified by measuring the percentage overlap between pre-operative targets (scar and mapping) and post-ablation areas confirmed by mapping, and post-explant TTC staining and histology.
The expected benefits—allowing concomitant VT ablation, improved consistency, shorter procedures, fewer repeat ablations—translate into both clinical and economic value. Though regulatory and compliance efforts for clinical translation are significant, they are minor compared to the costs of repeat ablations, prolonged procedures, and hospital stay/visits—especially once integrated into routine practice. While the training and integration of the device into the surgical workflow may initially add time to the procedure, it is expected to reduce overall operative time over the long term—particularly if intraoperative mapping can be reduced, potentially eliminating the need for an EP team in the operating room.
This silicone-based approach can serve as a basis for the engineering and development of other customizable guides. The potential for further applications is vast, as this can be adapted to meet specific mapping and procedural requirements. For example, our group previously developed an epicardial guide for Brugada syndrome ablation, highlighting the arrhythmogenic substrate in the right ventricular outflow tract, based on electro-anatomical mapping data [21]. In addition, we have created guides for coronary artery mapping and optimal bypass target placement during coronary artery bypass grafting [22], and sinus node protection for inappropriate sinus tachycardia ablation. Furthermore, this approach could extend to other (non-)cardiac procedures for anatomical modeling, pre-operative planning, and surgical training.
Limitations and future considerations
Several limitations should be considered. As this was an early proof-of-concept study, the 3D-printed mold was fabricated from non-biocompatible PLA. Thus, biocompatibility testing, including in vitro cytotoxicity assessments as per ISO 10993–5:2009 (https://www.iso.org/standard/36406.html), as well as post-sterilization material characterization assessments are warranted for future research. Following, in vivo porcine experiments are needed on the beating heart to assess stability, fit, ablation efficacy, and electro-anatomic alignment more in-depth.