Introduction
Mechanisms of action
Health benefits
Dietary sources and products
Challenges and considerations
Conclusions
References
Further reading
Synbiotic diets combine probiotics and prebiotics to enhance gut microbiota balance, improve metabolic health, and support immune function through synergistic biological mechanisms. These formulations show promise in managing gastrointestinal, metabolic, and inflammatory conditions while emphasizing strain specificity and clinical validation.
Image Credit: Fuseass / Shutterstock.com
Introduction
Gut dysbiosis, which reflects an imbalance in the microbial composition of the gastrointestinal microbiome, increases the risk of developing metabolic and inflammatory diseases, thus emphasizing the importance of strategies that promote optimal gut health. Among these, functional food ingredients such as probiotics, prebiotics, and their synergistic combination, synbiotics, have gained increasing popularity worldwide.1
According to the World Health Organization (WHO), probiotics are live microorganisms that confer health benefits when consumed in sufficient amounts. Prebiotics are best defined (per consensus now adopted in recent reviews) as substrates selectively utilized by host microorganisms that confer a health benefit, rather than “stimulating the activity of probiotic bacteria.”15
Synbiotics combine probiotic strains, such as Lactobacillus and Bifidobacterium, with prebiotic fibers, including inulin or galacto-oligosaccharides, to enhance microbial survival and function. This concept has been updated to emphasize a mixture of live microorganisms and substrate(s) selectively utilized by host microbes that together confer a health benefit.4
Mechanisms of action
Combining prebiotics, like fructooligosaccharides (FOS), xylooligosaccharides (XOS), and inulin, with probiotic microorganisms like Bifidobacterium, Lactobacillus, Saccharomyces boulardii, and Bacillus coagulans enhances their viability as they travel through the acidic environment of the upper gastrointestinal tract. Prebiotics serve as a selective nutrient source, which enables these beneficial bacteria to persist and colonize more efficiently in the colon.1,2
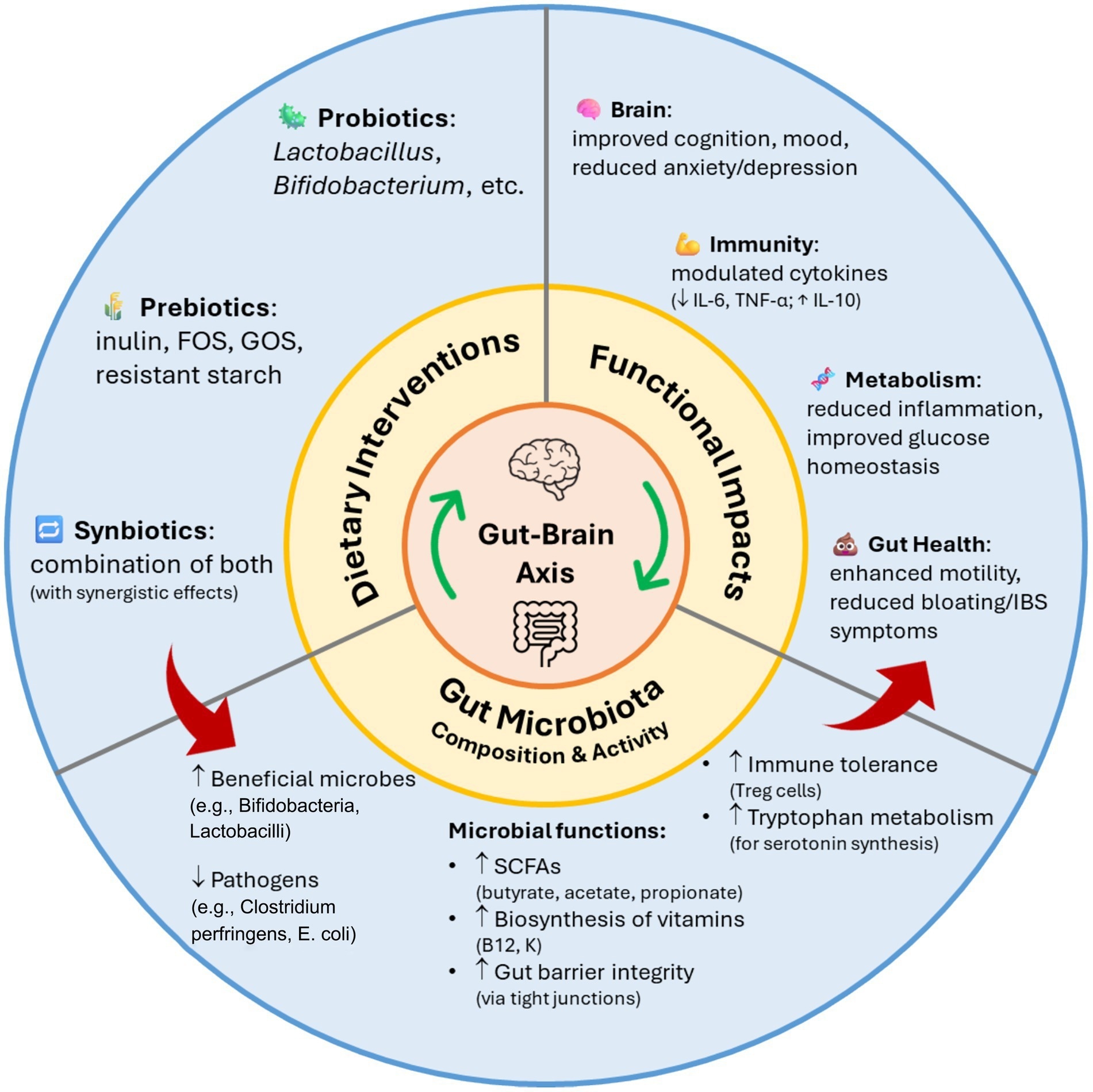
Impact of probiotics, prebiotics, and synbiotics on gut microbiota composition and function.10
Prebiotic fermentation also yields short-chain fatty acids (SCFAs), such as butyrate, acetate, and propionate, that strengthen epithelial barrier integrity, regulate intestinal pH, and modulate immune cell signaling. SCFAs serve as energy substrates for colonocytes and influence gut-associated lymphoid tissue (GALT), thereby enhancing mucosal immunity and suppressing inflammatory cascades.1,2
By fostering a balanced microbial community, synbiotics suppress the proliferation of pathogenic bacteria, such as Clostridium perfringens, Salmonella, and Escherichia coli, while promoting the growth of probiotic bacteria. This equilibrium strengthens the intestinal barrier, improves digestion, and aids recovery following antibiotic therapy.15
These interconnected mechanisms form the biological basis for the improvements in digestive, metabolic, and immune outcomes documented in clinical trials discussed below.
Science Animation – Synbiotics and Infant Health
Health benefits
Digestive and immune health
In a randomized trial in middle-aged adults, a synbiotic pairing of Bifidobacterium animalis subsp. lactis with FOS reduced days with abdominal discomfort and lowered circulating proinflammatory cytokines (for example, IL-6, IL-8, IL-17A, IFN-γ), although stool frequency and consistency improved in both synbiotic and placebo groups.4 Animal data also support immunomodulatory effects: yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus mitigated DSS-induced colitis and altered regulatory T-cell responses in mice.3
Together, these findings suggest that synbiotics may enhance mucosal immune balance and reduce inflammatory signaling, though human outcomes vary across formulations and durations.
Metabolic regulation
In 60 diabetic hemodialysis patients, a 12-week multi-strain synbiotic formulation, including L. acidophilus, L. casei, and B. bifidum, with inulin, decreased fasting plasma glucose, insulin, homeostatic model assessment for insulin resistance (HOMA-IR), high-sensitivity C-reactive protein (hs-CRP), and malondialdehyde levels, while increasing antioxidant capacity and glutathione.5 Similarly, women with gestational diabetes exhibited significant improvements in triglyceride (TG) high high-density lipoprotein (HDL) ratios after six weeks of synbiotic supplementation.6
Moderately hypercholesterolemic men consuming a fermented soy product with E. faecium CRL183 and L. helveticus for 42 days experienced reductions of 13-24% in total cholesterol, non-HDL cholesterol, and electronegative low-density lipoprotein (LDL) levels while preserving HDL.7 In people with obesity, Lactobacillus plantarum K50 reduced total cholesterol and triglycerides over 12 weeks.9 A 6-month multi-strain probiotic also lowered circulating endotoxin and inflammatory markers, with the most robust between-group effect observed for HOMA-IR.8
Mechanistically, probiotics convert cholesterol to bile acids and incorporate it into bacterial membranes, while prebiotics promote SCFA production to reduce serum lipid levels and systemic inflammation.9
These data support a modest but consistent metabolic benefit profile, primarily in lipid and glycemic parameters, contingent on strain composition and dose.
Gut-brain axis and mental health
Evidence syntheses indicate that probiotics, prebiotics, and synbiotics can influence the microbiota–gut–brain axis via neural, endocrine, immune, and metabolic routes; signals include SCFAs and neurotransmitter modulation. Reported benefits on mood and stress are promising but heterogeneous across strains and study designs.10
Thus, while mechanistic plausibility is strong, confirmatory clinical data are still emerging, and mental health outcomes remain an evolving research frontier for synbiotic therapy.

Bidirectional communication pathways of the gut-brain axis.10
Dietary sources and products
Synbiotic foods integrate probiotics and prebiotics into a single matrix to create functional products that promote gut and immune health. Yogurt fortified with inulin, a prebiotic fiber derived from chicory root or Jerusalem artichoke, enhances the metabolic activity of Lactobacillus and Bifidobacterium to support their survival during digestion. Formulations can also include plant polysaccharides, such as aloe vera gel, to support probiotic viability and antioxidant capacity, while maintaining an acceptable sensory profile under refrigerated storage conditions.11
Kefir, a fermented milk or water-based drink containing lactic acid bacteria, bifidobacteria, and yeasts retained in kefiran, a polysaccharide matrix, represents a natural synbiotic. Kefiran acts as a prebiotic while simultaneously conferring antioxidant and antimicrobial effects. Recent reviews summarize benefits spanning gut health and metabolic endpoints, though clinical evidence varies by product and protocol.12
 Image Credit: Marcus Z-pics / Shutterstock.com
Image Credit: Marcus Z-pics / Shutterstock.com
Tempeh, a fermented soy product, combines prebiotic fibers with microbial fermentation. Although heat inactivates live probiotics, residual microbial components known as paraprobiotics continue to support gut and immune modulation.13
Fortified functional foods, such as yogurt, beverages, snack bars, and frozen desserts, are widely formulated with Lactobacillus rhamnosus, Bifidobacterium bifidum, and prebiotics like inulin, FOS, or GOS. Advances in microencapsulation technology further improve probiotic viability and shelf stability, thereby enabling convenient dietary strategies to maintain gut homeostasis and systemic immunity.14
These examples illustrate how diverse matrices can deliver both live microbes and fermentable substrates, offering flexible options for daily intake.
Challenges and considerations
Strain specificity is a critical factor that determines the effectiveness of synbiotic formulations, as the effects of probiotics are highly strain-dependent. A single strain may exhibit distinct benefits when administered alone as compared to when combined with other strains, with efficacy also variable across patient populations.
The effectiveness of synbiotic formulations is determined by the proportion of prebiotics and probiotics, amount consumed, and individual gut microbiome composition. Importantly, clinical improvements, such as reduced bloating or enhanced immune function, may not manifest for several weeks.2
In the United States, probiotics in foods and supplements are Generally Recognized as Safe (GRAS), whereas the European Food Safety Authority (EFSA) applies the Qualified Presumption of Safety (QPS) framework to these products. Unlike pharmaceutical drugs, dietary supplements are subjected to limited efficacy and safety testing. Consequently, product claims are often unsupported by rigorous clinical evidence.
Variability in label accuracy and viable counts can lead to inconsistent outcomes, and individuals with severe illness or immunosuppression may rarely experience adverse events.15
Consumers and clinicians should therefore match strain and dose to studied endpoints, verify product viability and storage conditions, and exercise caution in immunocompromised populations.
Conclusions
By enhancing probiotic survival, diversifying the gut microbiota, and improving nutrient handling, synbiotics may support digestive and immune function, contributing to the modification of metabolic risk. A growing body of clinical evidence supports benefits in specific contexts (for example, glycemic indices in dialysis or lipids in obesity), but effects remain strain- and product-specific, and heterogeneity across trials warrants cautious interpretation.4
When these mechanistic and evidence-based perspectives are integrated, synbiotics emerge as promising yet context-dependent tools for modulating dietary effects on gut health.
References
- Haque, S. A., Elias, M., Olqi, N. A., & Al Uraimi, T. (2023). Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics. Nutrients 16(22); 3955. DOI:10.3390/nu16223955, https://www.mdpi.com/2072-6643/16/22/3955.
- Pandey, K. R., Naik, S. R., & Vakil, B. V. (2015). Probiotics, prebiotics, and synbiotics- a review. Journal of Food Science and Technology 52(12); 7577. DOI:10.1007/s13197-015-1921-1, https://link.springer.com/article/10.1007/s13197-015-1921-1.
- Wasilewska, E., Zlotkowska, D., & Wroblewska, B. (2018). Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate sodium-induced colitis. Journal of Dairy Science 102(1); 37-53. DOI:10.3168/jds.2018-14520, https://www.sciencedirect.com/science/article/pii/S0022030218310505.
- Neyrinck, A. M., Rodriguez, J., Taminiau, B., et al. (2021). Improvement of gastrointestinal discomfort and inflammatory status by a synbiotic in middle-aged adults: A double-blinded randomized placebo-controlled trial. Scientific Reports 11(1); 1-12. DOI:10.1038/s41598-020-80947-1, https://www.nature.com/articles/s41598-020-80947-1.
- Soleimani, A., Motamedzadeh, A., Zarrati Mojarrad, M., et al. (2019). The Effects of Synbiotic Supplementation on Metabolic Status in Diabetic Patients Undergoing Hemodialysis: a Randomized, Double-Blinded, Placebo-Controlled Trial. Probiotics & Antimicrobial Proteins 11; 1248-1256. DOI:10.1007/s12602-018-9499-3, https://link.springer.com/article/10.1007/s12602-018-9499-3.
- Nabhani, Z., Clark, C. C., Goudarzi, N., et al. (2022). The effect of synbiotic supplementation on atherogenic indices, hs-CRP, and malondialdehyde, as major CVD-related parameters, in women with gestational diabetes mellitus: A secondary data-analysis of a randomized double-blind, placebo-controlled study. Diabetology & Metabolic Syndrome 14(1); 87. DOI:10.1186/s13098-022-00858-1, https://dmsjournal.biomedcentral.com/articles/10.1186/s13098-022-00858-1.
- Cavallini, D., C. U., Manzoni, M. S. J., Bedani, R., et al. (2016). Probiotic Soy Product Supplemented with Isoflavones Improves the Lipid Profile of Moderately Hypercholesterolemic Men: A Randomized Controlled Trial. Nutrients 8; 52. DOI:10.3390/nu8010052, https://www.mdpi.com/2072-6643/8/1/52.
- Sabico, S., Al Mashharawari A., Al-Dahgri, N. M., et al. (2019). Effects of a 6-Month Multi-Strain Probiotics Supplementation in Endotoxemic, Inflammatory, and Cardiometabolic Status of T2DM Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Clinical Nutrition 38; 1561–1569, DOI:10.1016/j.clnu.2018.08.009, https://www.clinicalnutritionjournal.com/article/S0261-5614(18)31351-7/fulltext.
- Sohn, M., Na, G. Y., Chu, J., et al. (2022). Efficacy and Safety of Lactobacillus Plantarum K50 on Lipids in Koreans with Obesity: A Randomized, Double-Blind Controlled Clinical Trial. Frontiers in Endocrinology 12, 790046. DOI:10.3389/fendo.2021.790046, https://www.frontiersin.org/articles/10.3389/fendo.2021.790046/full.
- Kezer, G., Paramithiotis, S., Khwaldia, K., et al. (2025). A comprehensive overview of the effects of probiotics, prebiotics, and synbiotics on the gut-brain axis. Frontiers in Microbiology 16; 1651965. DOI:10.3389/fmicb.2025.1651965, https://www.frontiersin.org/articles/10.3389/fmicb.2025.1651965/full.
- Ahmed, S., Noor, A., Tariq, M., & Zaidi, A. (2023). Functional improvement of synbiotic yogurt enriched with Lacticaseibacillus rhamnosus and aloe vera gel using the response surface method. Food Production, Process and Nutrition 5. DOI:10.1186/s43014-023-00153-0, https://fppn.biomedcentral.com/articles/10.1186/s43014-023-00153-0.
- Tingirikari, J. M. R., Sharma, A. & Lee, H. J. (2024). Kefir: a fermented plethora of synbiotic microbiome and health. Journal of Ethnic Food 11; 35. DOI:10.1186/s42779-024-00252-4, https://journalofethnicfoods.biomedcentral.com/articles/10.1186/s42779-024-00252-4.
- Subali, D., Christos, R. E., Givianty, V. T., et al. (2023). Soy-Based Tempeh Rich in Paraprobiotics Properties as a Functional Sports Food: More Than a Protein Source. Nutrients 15(11), 2599. DOI:10.3390/nu15112599, https://www.mdpi.com/2072-6643/15/11/2599.
- Yadav, M., Sehrawat, N., Sharma, A. K., et al. (2022). Synbiotics as potent functional food: Recent updates on therapeutic potential and mechanistic insight. Journal of Food Science and Technology 61(1). DOI:10.1007/s13197-022-05621-y, https://link.springer.com/article/10.1007/s13197-022-05621-y.
- Markowiak, P., & Śliżewska, K. (2017). Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 9(9); 1021. DOI:10.3390/nu9091021, https://www.mdpi.com/2072-6643/9/9/1021.
Further Reading
Last Updated: Nov 11, 2025