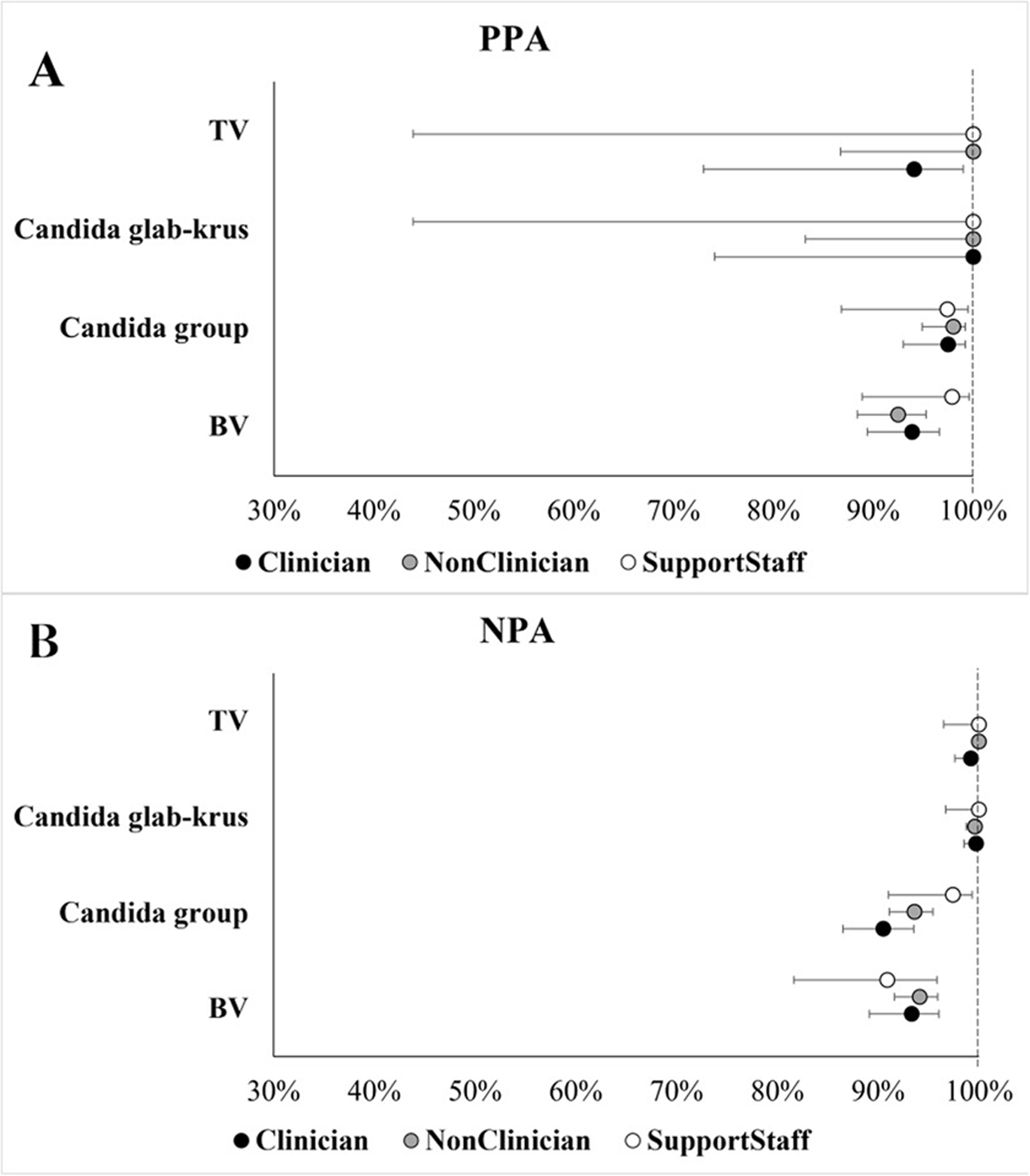
In this first-ever multi-site user-experience study for POC diagnosis of vaginitis, the MVP test demonstrated consistent performance across user types (P > 0.082) and users’ educational status (P > 0.050) based on PPA and NPA estimates for all 4 targets in the MVP test. User feedback showed that 15/19 (79%) of users found the Xpress System easy to set up, 18/19 (96%) found the MVP test instructions easy to follow, and all 19/19 (100%) of users responded that they “agree/strongly agree” that it was easy to perform the MVP test.
Highly sensitive molecular diagnostics historically have had a long turnaround time (hours which results in a practical time for the patient of days), required expensive equipment, and required skilled operators. POC NAAT-based diagnostic tests, particularly those with CLIA-waived designation, have the potential to lessen the time from collection to patient management, to improve accuracy of same-day treatment, and to minimize long term follow up healthcare costs. The Xpert® Xpress MVP test is the first CLIA-waived, FDA-cleared POC NAAT test designed to diagnose vaginal infections in symptomatic women within an hour with minimal hands-on time. Understanding the ease-of-use of these tests by operators with varying skill sets is important for effective implementation of testing strategies.
Our study was limited by the artificial nature of the testing since the performance of the assay was under evaluation and multiple comparators tests were being performed. In most cases, the tests were not performed in a manner that reflects the routine clinical workflow, which might involve a sample-first collection and testing process [5]. Fuller and colleagues found that by having patients collect samples for chlamydia/gonorrhea testing prior to engagement with a healthcare provider and beginning the test based on intake triage questions, the time to results was more offset in part by routine clinical wait times. In the clinic, they described the additional time for patients that resulted from a 90-minute test to be, on average, 46 min. Similarly, Gettinger, et al. found that a 30-minute chlamydia/gonorrhea test would add, on average, only 11 min to a visit in a very efficient clinic setting [6]. However, to utilize sample-first testing, means that the staff performing the testing may be different from the type of staff in this analysis and they may not be as comfortable with the process as the staff engaged in this research project. Additional research is needed into the implementation strategies that will best support utilization of POC tests such as the one used in this study.
Evaluation of a CLIA-waived test also impacted how new users implemented the MVP test. The absence of written competency checklist or verification of adherence to the IFU occurred prior to testing in the study reflects the practice for generalizable use and as such, our study findings may apply more broadly to the use of other POC tests as well. In contrast, users were only allowed to use the IFU and QRI for operation instructions whereas, in a real-world setting, they would have been able to contact the manufacturer for assistance.
Additionally, due to secondary nature of the analyses, the number of specimens in some of the user type categories and the educational level categories were small (large 95% confidence intervals) and hence the analyses were exploratory in nature. Thus, real-world implementation studies will be needed to assess ease of use for a variety of potential testers. This type of evaluation has been performed for chlamydia/gonorrhea testing, demonstrating the variability of performance based on the testing sites which highlights the importance of oversight and quality management to indicate when retraining or training updates may be necessary [3, 7]. Finally, it is important to note the we did not assess the cost implications of adoption because of the nature of this secondary analysis. This important factor in adoption and sustainability must be evaluated in future research efforts.