Study aims
Participants in AgeWISE-AP will be compared to a no treatment control group. There are three primary aims of the present study as well as a fourth exploratory aim.
-
Aim 1: To determine whether AgeWISE-AP increases older Veterans’ engagement in lifestyle factors that promote brain health. AgeWISE provides information about the differences between normal and diseased brain aging and lifestyle factors that contribute to brain health. The action plan (AP) component will use this foundation to collaboratively create an individualized brain health plan to increase Veteran engagement in brain healthy lifestyle activities. We hypothesize that AgeWISE-AP participants will demonstrate increased engagement in brain-healthy lifestyle activities compared to the control group.
-
Aim 2: To determine whether AgeWISE-AP improves psychological wellness. AgeWISE provides information about the relationship between cognitive aging and affective states (e.g., depression) and attitudes about aging, and teaches stress reduction techniques. The action plan (AP) will provide additional individualized supports to improve psychological well-being with personalized goals and lifestyle modifications (e.g., diet, exercise, sleep, cognitive stimulation). We hypothesize that AgeWISE-AP participants will show increases in perception of control over cognitive aging, meaning and purpose in life, quality of life, and self-efficacy, as well as improved attitudes toward aging and decreased loneliness, depression, and anxiety compared to the control group.
-
Aim 3: To determine whether AgeWISE-AP improves cognition. Cognitive strategies to improve cognition and functioning are presented and practiced over three AgeWISE sessions, with homework to improve generalization to day-to-day life. Engagement in brain-healthy lifestyle activities will be accomplished through the action plan (AP). We hypothesize that self-reported memory contentment, ability, and compensatory strategy use will increase. We also hypothesize that objective cognitive performance will be better for AgeWISE-AP participants compared to the control group.
-
Exploratory Aim 4: To determine whether AgeWISE-AP influences biomarkers of brain health using structural neuroimaging methods. We hypothesize that at one-year follow-up, AgeWISE-AP participants will have less volumetric decline in brain regions of interest compared to the control group.
Study overview
The current study is a Phase III randomized controlled clinical trial. The study will employ two arms: the AgeWISE-AP intervention arm and a no treatment control group arm.
AgeWISE-AP intervention arm
AgeWISE-AP includes engagement in the original 12-week, one hour per week AgeWISE group plus an additional 8-session individualized action plan. Group AgeWISE sessions include education, discussion, and in-class exercises designed to practice introduced skills. Out of session homework assignments are given weekly to promote generalization of training into daily life. Table 1 displays an outline and summary for the 12 sessions in the intervention (as seen in O’Connor et al., 2018 [52]). During week 9 of the AgeWISE group, Veterans will begin the novel AP component of the intervention.
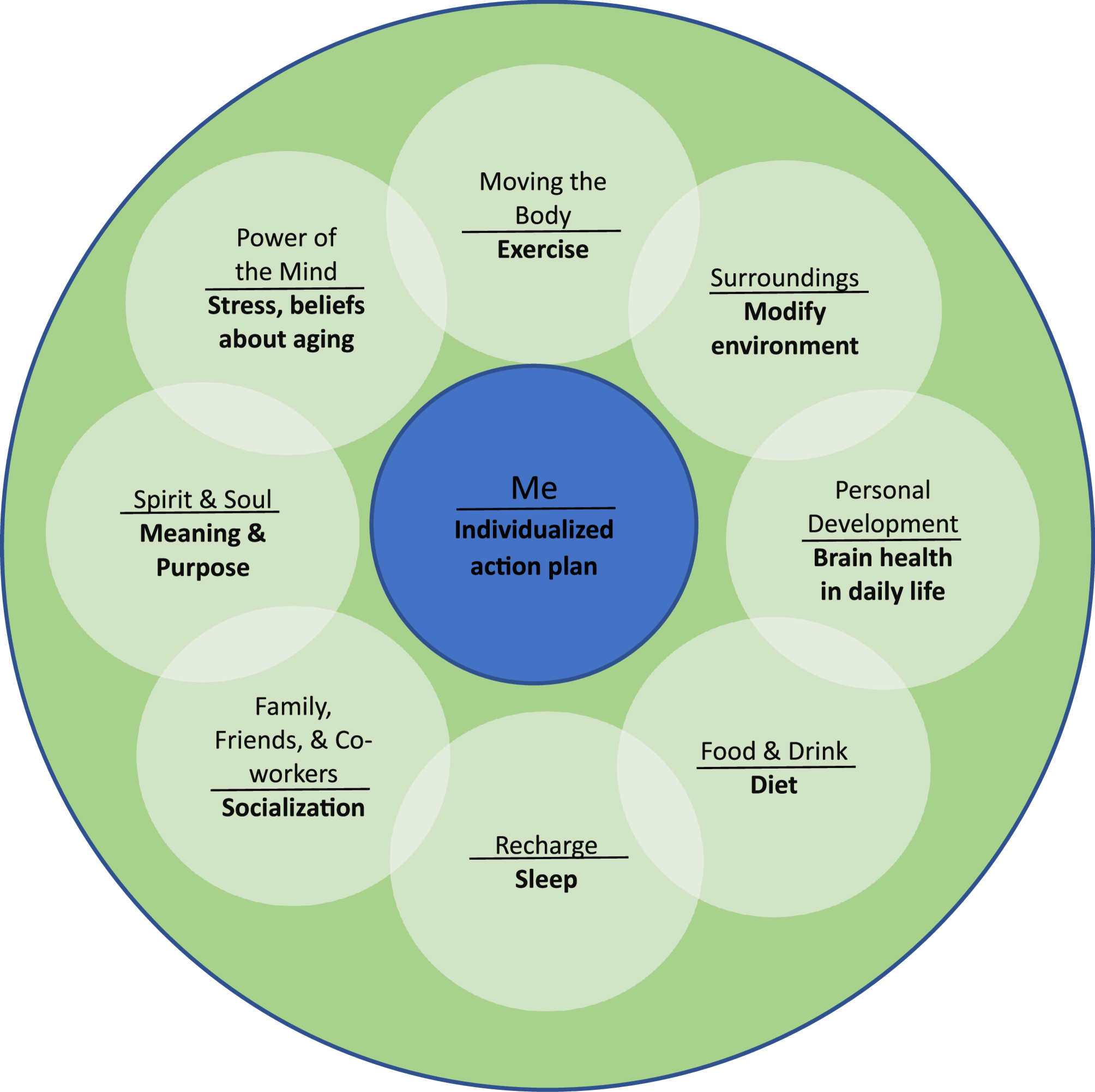
The AP component will be run by a doctoral level occupational therapist serving as the Brain Health Interventionist. The Brain Health Interventionist meets with Veterans individually, once a week over the first month (final 4 weeks of AgeWISE) to define at least three specific goals across lifestyle factors associated with dementia risk (exercise, diet, sleep, socialization, cognitive stimulation) and create an actionable plan to meet those goals. The Brain Health Interventionist then meets with the Veteran every other week (4 additional sessions for a total of 8 individual sessions) to monitor progress, identify barriers to progress, and revise the plan as needed. The Brain Health Interventionist uses the Whole Health Personal Health Inventory and Personal Health Plan worksheets, modified for the current study to focus on brain health, to help determine targeted goals for change. Motivational interviewing techniques are incorporated throughout individual sessions. The Brain Health Interventionist assists with coordination of referrals to existing programs within and outside of the VA system specific to Veteran-identified goals. For example, a Veteran who identifies increased exercise as a goal might be encouraged to join the VA GeroFit program or attend classes at their local YMCA. As needed, the Brain Health Interventionist may make the referral, provide the phone number for YMCA membership, print a copy of the class schedule, and help the Veteran brainstorm solutions to barriers such as transportation.
Control group arm
The control condition is a no treatment control. Although other control conditions could have been proposed, no treatment was chosen for this study for a number of reasons including (a) the desire to fully capture what occurs in the real lives of older Veterans, which typically involves no active treatment (minimal education and skills training related to cognitive aging concerns from other professionals may occur, as may self-generated acquisition of knowledge regarding cognitive aging), and (b) the desire to minimize the influence of increased socialization with other older Veterans having similar concerns, as we suspect that such interaction is one active ingredient of the intervention. Following study completion, control group Veterans will be invited to participate in the AP intervention as a clinical service.
Participants
A total of 128 Veterans will be recruited into the study staggered across Years 1 and 2 to ensure that the workload is manageable (an average of 16 participants will be recruited per study quarter). Recruitment takes place at the Bedford VA Healthcare System. All potentially eligible Veterans are asked if they would like to hear information about a study related to cognitive aging. Those who agree are given a study flyer and provided with a brief description of the study and procedures. Those who express interest are screened for eligibility and will complete the informed consent process and HIPAA authorization (see Table 2 for a schedule of enrollment, interventions, and assessments). During the informed consent process, participants are given the option of undergoing brain imaging at baseline and one-year follow-up for Aim 4. Participants that agree to neuroimaging undergo an additional informed consent process during the first of two brain imaging sessions, and complete a screening questionnaire specific to brain imaging safety. The informed consent processes occur in a private office setting and the information is presented both verbally and in written format. Veterans are asked to paraphrase informed consent information to ensure adequate understanding. Veterans are given ample time to ask questions about the study. After the informed consent, Veterans undergo a study screening that includes administration of the Montreal Cognitive Assessment (MoCA). Inclusion criteria for the present study includes Veterans ≥ 60 years old with concerns about brain aging who want to learn more about cognitive aging; and English speaking as all intervention materials are written in English. Exclusion criteria for the present study includes Veterans with impairment on a cognitive screening measure, as determined using a MoCA cutoff score for dementia of < 24 [53], or self or other reported diagnosis of a brain disorder affecting cognition such as Alzheimer’s disease, Parkinson’s disease, other dementia, major stroke, brain injury, or diagnosis of psychotic disorder such as schizophrenia; and active alcohol or substance abuse.
Procedures
After eligible Veterans complete informed consent, screening, and baseline measures for study inclusion, each is randomized to one of the two study arms: AgeWISE-AP (intervention arm) or the control condition. Randomization uses two computer-generated numbered lists; one for each condition. The lists are generated through a permuted block design. With this approach, each randomization list consists of two AgeWISE-AP assignments and two control condition assignments in random order. This approach not only guarantees balance in the two conditions, but also balances assignments due to the time of recruitment.
The manualized AgeWISE group classes are run by doctoral level psychologists within the Bedford VA Healthcare System’s Neuropsychology Department. The study team discusses any issues related to the intervention sessions during weekly meetings. The AP component is run by a doctoral level occupational therapist serving as the Brain Health Interventionist to increase older Veteran participation in lifestyle activities associated with healthy brain aging. The Brain Health Interventionist helps the Veteran complete the Whole Health Personal Health Inventory and Personal Health Plan worksheets, which were modified to focus on brain health, to determine personalized goals, create an actionable plan to meet goals, and provide assistance with program referrals as needed (4 initial weekly sessions, 1 h per week). The Brain Health Interventionist then meets with the individual Veteran every other week for the final 8 weeks (4 additional sessions, 1 h every other week, for a total of 8 AP sessions) to review goals, boost motivation through accountability check-ins, determine any barriers to behavior change, and modify and adjust goals accordingly.
The study has been approved by the VA’s Central Institutional Review Board (cIRB) and Scientific Review Committee. Participant recruitment is at a target rate of 1–2 Veterans a week, projected at approximately 16 Veterans per study quarter during months 4 through 27 (see Table 3). The Bedford VA Healthcare System is a Category 3 facility located in the north-west suburbs of Boston serving approximately 20,000 Veterans with the bulk of clinical services in mental health, primary care, and geriatrics and extended care. Participants will be recruited from neurological, psychological, geriatric, and primary care services.
For Aims 1 through 3 (Primary Aims), the baseline evaluation takes participants an average of 1.25 h (1 h and 15 min) to complete the following measures, including collection of the demographic data (see below); sessions can be broken into two sessions as needed to minimize any fatigue. Follow-up evaluations take place immediately after the AgeWISE-AP intervention has ended (immediate post intervention), 3-months post intervention, and 6-months post intervention (i.e., 1 year after enrollment) and take an estimated hour and a half to complete. Structural imaging data is collected at baseline and again at one year, taking an estimated hour and a half to complete, with 30–45 min preparation for scanning and another 45 min of time spent in the scanner.
Assessments
The outcome measures for this study were chosen to inform our specific aims. We seek to investigate whether AgeWISE-AP boosts older Veterans’ engagement in lifestyle activities that promote brain health (Aim 1) and improves psychological wellbeing (Aim 2) and cognition (Aim 3) compared to a treatment as usual (no intervention) control group. We will additionally explore whether there is an intervention effect on biomarkers of brain health using structural neuroimaging methods (exploratory Aim 4). The following measures will be used to test the hypotheses associated with each aim. A detailed description of each measure is provided below. A demographic questionnaire will be administered only at baseline that captures basic data including age, gender, race, educational history, occupational history, medical history, and psychiatric history.
Outcome measures
The following instruments will be administered at baseline (pre-intervention), immediately post intervention, and 3- and 6-months post intervention (see Table 4).
Aim 1: lifestyle factors
Community Healthy Activities Model Program for Seniors Questionnaire (CHAMPS) [54] is a 41-item scale that explores the frequency and duration of light, moderate, and vigorous physical activities assessed using weekly frequency and duration. Test-retest reliability was acceptable (ICCs 0.56-0.70).
Single Item Global Quality of Life Scale (SIG-QOL) [55] is a single item question measuring quality of life using a visual analogue scale ranging from 0 to 100. The single-item scale has high test-retest reliability (ICC was 0.87) and has shown high correlations with multi-item scales.
Health-Promoting Lifestyle Profile II (HPLP II) [56] is a 52-item questionnaire composed of six subscales including health responsibility, nutrition, physical activity, stress management, interpersonal relations, and spiritual growth. The English version of HPLP-II has shown high internal consistency and test-retest reliability.
Global Sleep Assessment Questionnaire (GSAQ) [57] is 11 items covering mood, life activities, and medical issues as they relate to sleep and symptoms associated with disorders of sleep. The GSAQ test-retest reliability reported the ICC ranged from 0.51 to 0.92.
Aim 2: psychological wellbeing
General and Memory Specific Control Beliefs Scale [58] measures perceived control over cognitive health. The scale is composed of two sets of items focusing on general and memory-specific control beliefs.
Philadelphia Geriatric Center Morale Scale (PGCMS) [59] is a 17-item scale measuring dimensions of emotional adjustments in persons aged 70 to 90. It provides a multidimensional approach to assessing the state of psychological wellbeing and perceived morale using three factors: agitation, attitude toward own aging, and loneliness dissatisfaction. Test-retest reliability ranged from 0.91 after five weeks to 0.75 after three months.
The NIH Toolbox Meaning and Purpose Short Form [60] is an 8-item, form that assesses the degree to which participants feel their lives matter/make sense. Each item is rated on a 5-point Likert scale with responses ranging from “strongly disagree” to “strongly agree” and from “not at all” to “very much.” The internal consistency is high (Cronbach alpha was 0.92).
Patient Health Questionnaire-9 (PHQ-9) [61] is a reliable and valid (in a variety of patient populations) multipurpose instrument for screening, diagnosing, monitoring, and measuring the severity of depression.
Generalized Anxiety Disorder 7-item (GAD-7) Scale [62] is a commonly used measure of anxiety with 7 total items with high internal consistency (Cronbach alpha was 0.92) and test-retest reliability (ICC was 0.83).
General Self-Efficacy Scale (GSE) [63] is a 10-item psychometric scale that assesses optimistic self-beliefs to cope with a variety of difficult demands in life. Empirical evidence suggests that the GSE is a reliable and valid unidimensional measure across different cultural contexts.
Aim 3: cognition
Multifactorial Memory Questionnaire (MMQ) [64] is a measure constructed to reflect aspects of memory that are potentially amenable to clinical intervention. The Contentment subscale contains 18 statements that assess emotions and perceptions about current memory ability including anxiety, embarrassment, and irritability. The Ability subscale contains 20 items phrased as memory failures in everyday memory situations (e.g., forgetting an appointment). The Strategy subscale measures self-reported cognitive strategy use. The MMQ has been shown to have adequate content validity, test-retest reliability (0.86-0.93), internal consistency (0.83-0.95), and convergent and discriminant validity.
Montreal Cognitive Assessment (MoCA) [65] is a screening instrument that assesses multiple cognitive domains with total score ranging from 0 to 30 points, and a cut score of 24 has demonstrated very good specificity (by correctly identifying 87% of healthy participants) and excellent sensitivity when differentiating Mild Cognitive Impairment (90%) and Alzheimer disease (100%) from healthy comparisons. Test-retest reliability (patients tested 35 days apart) was high, with an intraclass correlation coefficient of 0.92. The internal consistency was also found to be high (Cronbach alpha on standardized items = 0.83).
Data collection and plan
Research staff will be trained by the PI and responsible for all baseline and follow-up evaluations. At each assessment, staff will schedule the next assessment, check current contact information, and get updates on the Veterans’ status to promote retention. Research staff are responsible for entering all data into VA Research Electronic Data Capture (REDCap), a HIPAA-secure, web-based data collection and management platform managed by the VA. The PI will perform monthly audits on the database beginning after baseline data entry to ensure that data entry timelines are on target. Drs. Tripodis and Frank will only have access to de-identified data. Drs. Tripodis and Frank will assist with analysis after data collection and at each follow-up time point. Drs. O’Connor and Moo will meet monthly with Drs. Tripodis and Frank to review data as it is analyzed.
The statistical analyses will be broken down by study aim. For study aims 1 through 3, linear mixed regression models will be used to test the effect of AgeWISE-AP. The models will include linear effects of time with random intercepts and slopes for time. The dependent variables will include baseline measures and all the scores in all the follow-up visits. The primary models, run in all participants, will also include the following predictors: baseline age, sex, education (years), time (years from baseline), and the interaction (cross-product) of each predictor with time. For these models, the intervention x time interactions, reflecting differences in slopes between the intervention and the control group will be of primary interest. We do not expect any missingness in baseline covariates, and imputations will not be performed for longitudinal models since linear mixed effects models assume missingness at random. Factor analyses will be conducted to assess the validity of our approach (i.e., measurement structure and invariance). For power calculations, we used a simulation-based approach with an alpha of 5%, and an assumed participant dropout rate of 15% over the follow-up period. Our proposed sample size of 64 participants per group (128 total) will allow us to detect at least an 8% group difference in all our main outcomes over 6 months, with at least 80% power.
For exploratory Aim 4, we will acquire structural imaging data at baseline and one year on a subset (15 per group) of participants to determine volumetric changes in regions of interest. Neuroimaging data will be acquired on a 3 T Siemens Magnetom Prismafit scanner (Trio-upgrade). Prismafit data will be collected with a 20‐channel head coil. Two‐high resolution whole‐brain T1‐weighted images using Magnetization‐Prepared Rapid Gradient Echo (MP‐RAGE) volumes (TR/TE = 2.53 s/3.35 ms, flip angle = 7°, 1 mm isotropic) will be acquired. Scans will be 3D sagittal acquisitions with 176 contiguous slices (imaging matrix = 256 × 176, in‐plane resolution = 1 mm, slice thickness = 1 mm). Vertex‐wise general linear models will be performed using FreeSurfer to examine longitudinal changes in regional cortical thickness and volume throughout the cerebral cortex and subcortical gray and white matter in the intervention and control groups.
Study timeline
The proposed study timeline is outlined in Table 2. The first 3 months of the project (Year 1, Q1) were dedicated to study start up activities, including staff hiring and training, designing recruitment materials, printing study data collection packets and AgeWISE group manuals, and developing the manual and workbook used by the Brain Health Interventionist. Recruitment and baseline data collection began in Year 1, Q2 at an anticipated rate of 1–2 Veterans a week, projected at approximately 16 Veterans a quarter. Veterans are randomly assigned to AgeWISE-AP or control (n = 8 intervention, n = 8 control each quarter). The AgeWISE-AP intervention (20-weeks total), consisting of 12 weeks of the AgeWISE group and 8 sessions (held over 12 weeks) of the individualized AP, will began in Year 1, Q3, with months 7–9 (Q3) engagement in the first AgeWISE group and months 9–11 (Q3, Q4) engagement in the first AP. Approximately 8 AgeWISE-AP intervention groups will run in total, beginning each quarter thereafter (Y1 Q4 through Y3 Q2) comprising approximately 7–8 Veterans each for a total of 64 intervention Veterans, meeting the recruitment goals based on power calculations. Three-month follow-up data collection will begin in Year 2, Q1, and 6-month follow-up data collection will begin in Year 2, Q2. All data will be collected by Year 4, Q1, month 3. Year 4 Q1 and Q2 will be devoted to final data analysis, manuscript preparation, and submission, with Year 4 dedicated to grant preparation to further develop AgeWISE-AP with the goal of establishing mechanisms for sustained intervention adherence. We will seek funding opportunities looking to examine the effectiveness of rehabilitation interventions that include Veteran self-management components and/or clinician-directed adjunct approaches that allow for longer term monitoring of adherence and functional outcomes in participants.