The present study reveals the utility of going beyond model-driven (i.e., GLM or multiple regression) contrast-based analyses of task fMRI data. Voxel-wise GLM analysis applies the same a priori specified model of the brain’s temporal response at every voxel, with resulting contrast maps (e.g., faces > shapes) reflecting an aggregate map comprised of any voxel significantly activated by the task, according to the a priori model. As shown in previous work7, this map vastly underestimates the extent of task-relevant neuronal activity, in part because brain regions and networks with temporal responses to the task that differ from the a priori model time courses will not be identified, and in part because the common subtraction paradigm to generate a contrast map removes brain activation common to both conditions. This neglects the dynamic relationships between brain areas and networks. Further, even if a subtraction map reflects a more spatially distributed pattern of brain activation, a GLM-based contrast map is an aggregate brain activation map that can reflect activity of an undifferentiated mix of temporally distinct networks. Here, by using tICA to resolve dynamic and temporally distinct task-related activity and networks, we uncover an appreciably larger-than-previously-considered engagement of the brain by the EFMT: 74% of cortex and circumscribed subcortical regions, including the amygdala and cerebellum.
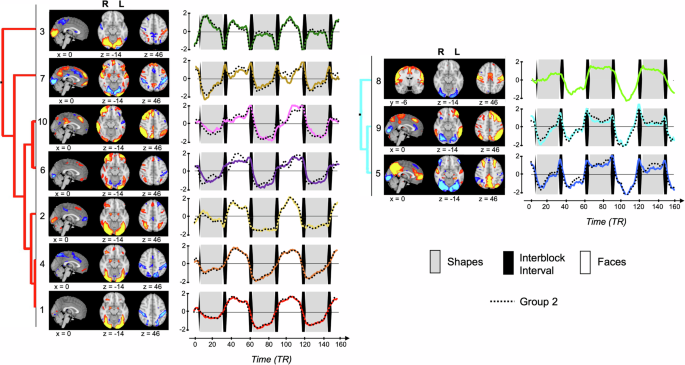
We found that EFMT-recruited brain networks with greater activity during face matching relative to shapes (i.e., Networks 1, 2, 3, 4, 6, 7, and 10) have diverse temporal activation patterns, likely reflecting distinct aspects of task performance. For instance, Network 10 activity ramped up during face blocks, while Network 2 activity remained more constant during face blocks. Several networks also displayed changes in their activation patterns across the course of the task run. For instance, Network 3 displayed strong activation during the first shape block, but little to no activation during subsequent shape blocks. Such differences between early and later blocks were observed for multiple networks, despite our focus on the second task run, suggesting ongoing impacts of learning and novelty on brain network activation within runs, even after a full run of practice. These within-block and within-run network activation changes (Fig. 1) identified using the tICA data-driven approach are not captured by standard voxel-wise GLM analyses, which model each task condition as a block of constant activity convolved with a hemodynamic response function and may reflect overlooked (or difficult to model) effects of practice, habituation, or fatigue. Overall, tICA’s use of fine-grained temporal information identified a richer-than-expected tapestry of concurrent neurobiological processes recruited by the EFMT, distinguishing brain networks with diverse activation patterns and with distinct relations to task performance and general cognition.
The 10 EFMT-recruited networks identified by tICA involve the interaction of visual association cortex with different sets of non-visual brain regions in distinct temporal fashions. These diverse interactions are supported by the multifaceted anatomical connections between the visual cortex and the rest of the brain. Kravitz et al.36 detail the ventral visual network’s projections to at least six distinct areas that support different cognitive functions, including an occipitotemporal-amygdala pathway involved in detecting and processing emotionally salient stimuli and an occipitotemporal-VLPFC pathway supporting object working memory. Networks 2 and 6 show extensive recruitment of both the occipitotemporal-VLPFC pathway and the occipitotemporal-amygdala pathway. However, their time courses suggest distinct roles in task performance. Network 2 remains active throughout face blocks, peaking mid-block, suggesting its engagement of working memory processes that support maintenance of the task rule set despite the presence of salient emotional stimuli, supported by DAN-mediated spatial executive attention. On the other hand, Network 6 activity increases over the course of each face block and is distinguished from Network 2 by the suppression of medial visual networks engaged during low-level processing of visual stimuli; as such, Network 6 may support the focus of visual attention as the interference load created by the emotional faces accrues across the block.
Notably, Network 2 also had the highest feature importance for predicting emotion interference, showing high subject loadings on Network 2 predicted high levels of emotion interference across samples. Network 10 was also significantly and reproducibly associated with individual differences in emotion interference during the EFMT as well as with general cognition across both groups. Both networks are preferentially activated during face blocks, but with distinct time courses (Network 2 activity peaking in the middle of face blocks and Network 10 activity peaking at the end of blocks), suggesting distinct roles of each network in the emotion regulation process. Unlike Network 2, Network 10 showed increased activation over the course of the faces blocks, activating most strongly within the second half of each of these blocks. While also containing lateral visual areas, Network 10 primarily resembled a right-lateralized FPN component, suggesting a role for this network in top-down executive control, which may become increasingly recruited over the course of the more challenging task blocks. This interpretation is bolstered by the observation that working memory task performance was a top predictive feature of Network 10 loadings. Network 5 (DMN + contextual association network) was also significantly associated with emotion interference, but in contrast to Networks 2 and 10, it was preferentially activated during inter-block intervals and suppressed during face blocks, which aligns with the DMN’s role as a task-negative network. This suggests that the capacity to suppress internally-focused thoughts during active periods of the task, and/or the ability to prepare for upcoming blocks during rest periods between task conditions, can also impact key aspects of task performance.
Even though individual differences in the recruitment of the remaining seven networks were not robustly associated with variability in emotion interference, they almost certainly play roles in supporting other cognitive aspects of task engagement and completion, as supported by the significant relations between several of these networks and general cognition (Fig. 7). The emotional face-matching condition and shape-matching (control) condition differ in several aspects besides emotion content that require engagement of multiple cognitive processes. First, the face stimuli are considerably more complex than the ovals used in the shape-matching condition, requiring modulation of focus and cognitive effort. Further, visual processing of human faces by other humans employs specialized brain mechanisms that enable rapid holistic assessment of facial identity and emotion37, requiring recruitment of distinct visual processing streams by the two conditions. Finally, the emotional face condition requires early attentional screening and later downregulation of salient yet extraneous emotional information to focus on the task-relevant aspects of the stimulus (i.e., matching the face identity). Each of these cognitive aspects of the task likely follows distinct time courses and is differentially impacted by practice, fatigue, repetition priming, and habituation.
For example, of these networks, 1 and 4 show increased engagement of the ventral visual stream, amygdala, and VLPFC in conjunction with deactivation of DAN and the premotor network. The negative spatial component of Network 4 also resembled the action mode network38, posited to activate during goal-directed and externally focused tasks. As such, each network is situated to process emotionally salient stimuli and modulate attention. These networks may, in part, act as complements to Network 2 to support spatially-focused attention within the matching task, providing concurrent suppression of attention to non-relevant areas of the visual field.
The positive component of Network 9 showed significant overlap with both DMN and FPN, although in contrast to Network 10 (which was associated with emotion interference and cognition), the FPN regions of Network 9 were left-lateralized. Network 9 also dramatically differed from Network 10 in its time course, showing suppression early in faces blocks as well as early in the first shapes block, which decreased over the course of these blocks. Activation of Network 9 occurred primarily within the inter-block interval, suggesting this executive network might play a role in task switching. The time course of Network 5 closely resembled that of Network 9 despite limited spatial overlap. Network 5 primarily overlapped with the DMN, which is activated during unconstrained resting state periods in the absence of goal-directed activity. As such, suppression of Network 5 during the first shapes task block and the faces blocks likely reflects the need for increased task focus during these periods and a consequent reduction in internally-focused or self-reflective cognitive processes. The increased activation of this network during the inter-block intervals may likewise result from a temporary increase in such internal reflections.
For Networks 3 and 7, which were rapidly activated or suppressed during the between-block transitions, activation may reflect the brief rest period, the presentation of the written instruction card, and preparation for a change in task requirements. The overlap with the language network in both cases suggests that the written instructions do play a role in the recruitment of these networks. The recruitment of DMN nodes and suppression of premotor and somatomotor cortex within Network 7, along with deactivation of visual networks and DAN during the intertrial interval in both networks, may reflect the temporary reduction in task demands. The recruitment of the parietal memory network during these intervals in Network 3 may be due to the need to recall general task instructions. In addition to the intertrial period, these networks were also differentially engaged during the faces and shapes conditions, though the engagement of Network 3 during the first shape block resembled that observed during subsequent faces blocks, and the suppression of Network 7 during shapes blocks decreased over the course of the run. These patterns converge to suggest roles for these two networks in the general recruitment and redistribution of attentional resources during the task.
Perhaps most surprisingly, despite the EFMT’s clear recruitment of multiple brain networks and regions implicated in emotion processing and internal affective state (e.g., amygdala), none of the EFMT networks reflected individual differences in internalizing/negative affect or well-being/positive affect. This coincided with the absence of the subgenual cingulate, a brain region centrally linked to negative affect and depression26,27, as a node in any of the EFMT-recruited networks. This was particularly striking given the involvement of nearly 75% of the cortex in one or more of these networks. One possible reason for the absence of subgenual cingulate in these networks is the known fMRI signal dropout in this brain region due to its proximity to tissue-air boundaries39. However, other brain regions known to experience similar signal dropout were detected here (e.g., fusiform gyrus)39. Moreover, other studies of related in-scanner tasks that engage emotion interference have also failed to reliably detect subgenual cingulate activity40,41. Together with convergent findings from the systematic review of the EFMT literature13 and other recent studies42,43, our findings suggest that while the EFMT may engage emotion-related processes, it may not be salient, challenging, or evocative enough to be a truly incisive tool for assessing individual differences in the NVS constructs. Notably, although several large-scale neuroimaging studies include this task as an RDoC NVS probe, the task is actually not listed in the RDoC matrix as a standard probe for the NVS.
There are some limitations to the present study. First, while tICA is a purely data-driven technique, its use involves some subjective decision points as described in the Methods, including the choice of model order and the classification of noise and signal components. Second, while the tICA identified 10 task-related networks and their temporal profiles, the task performance data that are available are somewhat limited for interpreting the task components that may be engaging each network. Probing the roles of each of these networks by linking to more detailed performance measures will be an exciting new direction for research. A second limitation of our study is that the participants in the HCP study were generally healthy young adults, with no or only sub-clinical levels of anxiety, depression, and drug use. This may limit our ability to link brain networks to individual variability in negative affect. However, our findings are consistent with the review by Savage et al.13, which also found no consistent relationship between brain activation in primarily emotion-processing brain areas during EFMT and mental health disorder diagnoses, including major and bipolar depression, anxiety-related disorders, and obsessive compulsive disorder, among others. But given the wealth of insights into brain dynamics from tensor ICA, exploration of this task in clinical populations using the tensor ICA approach may reveal relationships between brain network dynamics and clinical measures.
In summary, we used tICA to identify and characterize spatiotemporal features of brain network processes recruited by the EFMT, revealing a rich dynamic landscape of brain activity that has not been observed using conventional contrast-based analyses of this important task. Our findings call into question the EFMT’s suitability to evoke brain activity that distinguishes individual differences in NVS function in healthy young adults, although more work is needed to determine whether brain dynamics during EFMT is of utility in clinical populations.