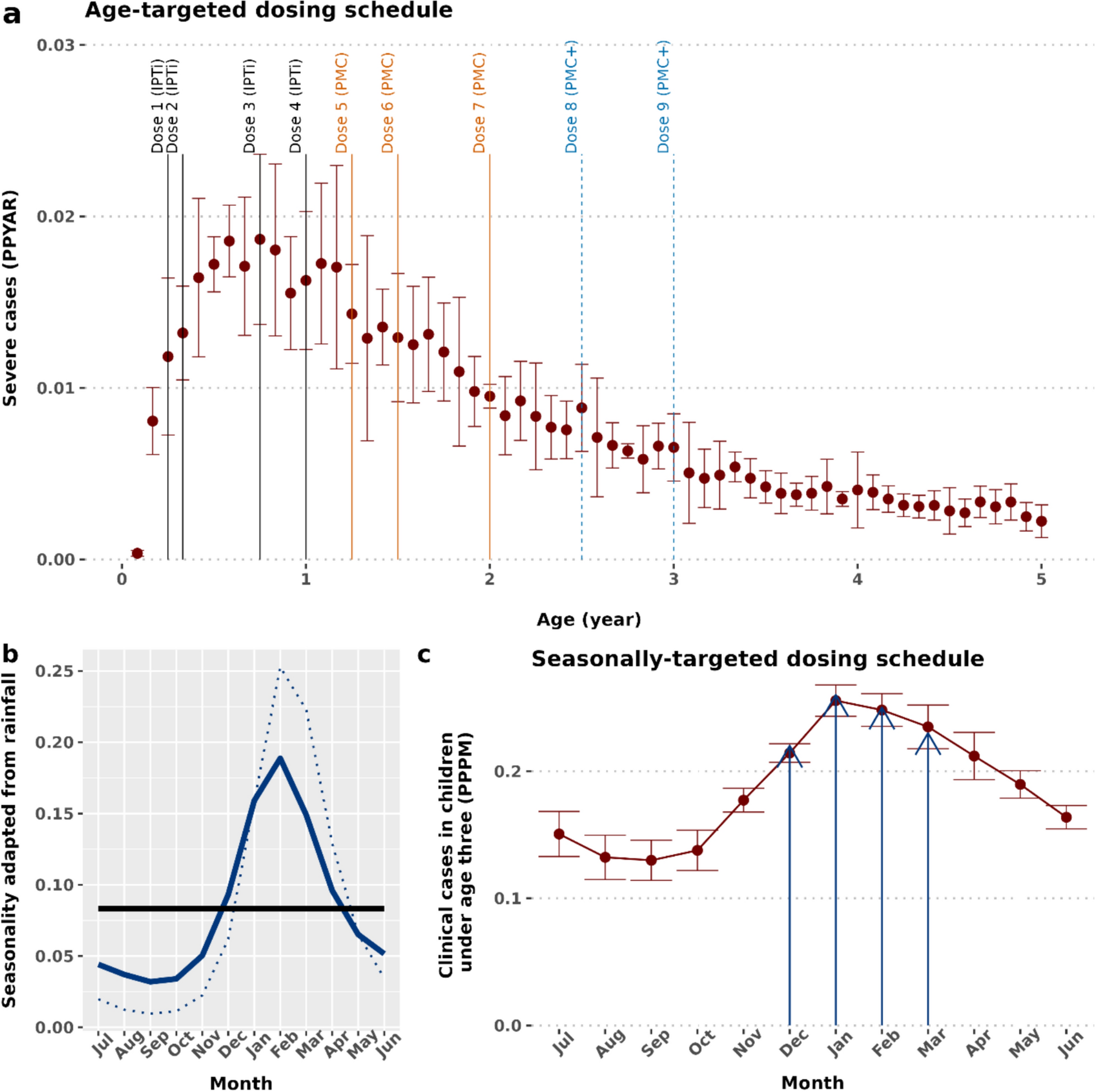
Optimized chemoprevention strategies in sub-perennial malaria transmission settings have received little attention. This study aimed to initiate multi-stakeholder dialogues to support chemoprevention guidelines for these settings by providing estimates of the potential public health impact and highlighting missed opportunities to protect vulnerable children. A validated open-access malaria model [17, 21] was used to explore the potential public health benefits of complementing current PMC recommendations with additional seasonally-targeted monthly SP doses during the high-risk transmission period. Since, there is no practical experience deploying mixed malaria chemoprevention to children, this study aimed to generate preliminary, quantitative evidence to inform the planning of new dosing strategies. Furthermore, enhancing the public health impact of PMC will be beneficial in ensuring a wider uptake of this historically less utilized yet efficacious and safe intervention across recommended settings that also include sub-perennial seasonality [1]. These model-driven estimates of the potential impact, benefits or risks are anticipated to support conversations on targeted chemoprevention in these settings, as well as support plans to generate empirical safety, feasibility, and impact data through pilot implementation studies.
Overall, the model assumptions and simulated parameter ranges, including access to case management, reflect sub-Saharan African settings, where PMC is currently implemented. A proposed hybrid malaria chemoprevention (HMC) strategy to cover children up to 24 months of age was modelled, aligned with the WHO’s PMC recommendations and consistent with experience to date [1]. However, since children remain vulnerable to severe malaria, and additional EPI contacts likely remain available up to 36 months of age, an age-expanded HMC + schedule was also assessed. In addition, if these additional EPI contact points were utilized as malaria chemoprevention touchpoints, they may improve access to other vaccines or health services. Different SP-sensitivities were assumed to account for resistance and ranges of access to case management and coverage levels to predict likely public health impact across example implementation scenarios and archetypal transmission settings. The HMC + included age-expanded PMC +, which demonstrated a likely increase in net public health benefits and cost-effectiveness than current PMC [23]. The monthly SP doses were given during the rainy season, combined with PMC or PMC + throughout the year, to prevent interruption of current practices. A maximum of seven or nine PMC or PMC + doses spread over two to three years was considered, aligned with the WHO guidelines on PMC dosing frequency [1]. However, some PMC doses during the rainy season would likely be missed (i.e. replaced with seasonally-targeted doses) to ensure a one-month gap between any two SP doses. These schedules were modelled over a wide range of prevalence settings (PfPR2–10 5–70%) in a combination of different drug sensitivity, healthcare strength (i.e. varying access to case management), and coverage assumptions. As the mode of delivery for seasonally targeted dosing remains unknown (e.g. extended capacity within EPI, CHW delivery, or an SMC-like campaign), the same coverage level was assumed for both age- and seasonally targeted delivery. Full coverage was simulated to estimate both maximum impact and the worst-case scenario in terms of delayed malaria. In this explorative study, only one reduced coverage level was presented to illustrate the potential impact on incidence and effectiveness compared with full coverage. A per-cycle 80% coverage was assumed for both PMC and seasonally targeted SP, in line with WHO vaccination targets to reflect EPI-based PMC [30, 31] and previous SMC modelling studies [20, 32] informed by implementation study data [33]. Chemoprevention coverage is known to vary widely, from over 80% in some SMC programmes [34] to around 50% for former IPTi delivery [35]. Also, given limited PMC uptake, there are currently insufficient estimates of its per cycle coverage. Therefore, implementation studies will be crucial to understand the preferred mode of seasonally-targeted SP delivery, and to monitor cycle coverage and adherence for both age- and seasonally targeted SP.
Model estimated efficacy against clinical, and severe cases, and the likely mode of parasite life stage activity of SP was validated to empirical data from randomized controlled trials and to a recent meta-data [5, 27] as described previously [23]. Both HMC and HMC + were predicted to substantially increase protection by averting clinical and severe malaria burden compared to the current PMC alone. This is due to the greater chances of getting an SP dose, through additional seasonal dosing, during a higher-risk period. In contrast, unless a child’s age aligns with the timing of high-risk periods, PMC does not ensure adequate protection.
The results indicated increased protection and maintenance of effectiveness in modelled partially SP-resistant settings, albeit with slightly reduced total malaria averted, also in line with earlier findings [21, 22, 29]. Although, systematic reviews have confirmed that the effect of SP resistance is modest on the effectiveness of chemoprevention [29], the protection might further reduce in settings with more resistant parasite genotypes [20]. This implies that it would be prudent to monitor the evolution and spread of drug resistance by genetic biomarker surveys following continued and new chemoprevention. A recent case–control study found that a higher malaria burden may also be attributed to suboptimal drug concentration, possibly caused by missed doses rather than drug resistance [36]. Thus, alternative drug candidates must be carefully investigated to safeguard their chemoprevention benefits without compromising treatment options.
Malaria outcomes from the simulations for children older than those covered by the proposed HMC or HMC + (up to five years of age) were analysed to assess any post-intervention effects, across access to case management levels reflecting sub-Saharan African settings [23,24,25]. The results demonstrated a larger positive net impact (cumulative cases by age during, and in the post-intervention period) of both HMC and HMC + compared to PMC alone, thus reducing the potential delayed malaria burden. This positive net impact of HMC and HMC + remained higher than PMC alone, also for scenarios with modelled lower coverage. Notably, improved and reliable access to treatment will be necessary to ameliorate any increased risk of malaria and manage severe malaria cases after children are no longer protected by chemoprevention as is the case for any child who is no longer using an effective form of malaria prevention) [23]. This is aligned with the WHO emphasis on strengthening healthcare systems. The increased impact of these chemoprevention strategies not only eases the demand on malaria treatment but also strengthens the capacity to prevent and treat other health needs [37]. Although, these results alleviate concerns about delayed malaria, it will be important to monitor age-incidence relationships and the net impact following expanded chemoprevention programme implementation in empirical setting to confirm these model-driven findings.
To avoid potential safety concerns related to multiple dosing, the HMC or HMC + schedules were designed to restrict the number of doses each child received, ensuring a one-month gap between any consecutive age- or seasonally-targeted SP doses. However, it will be necessary to integrate stakeholder, community, and implementation perspectives in planning the timing of hybrid dosing strategies, including the total number and timing of both age- and seasonally-targeted doses to understand the feasibility, acceptability, safety, and effective implementation and communication [1].
Additionally, the uncertainties related to changing climate conditions were taken into consideration. For example, less rainfall in some years may lead to less seasonal variation in malaria transmission. Thus, the potential benefit of rolling out HMC or HMC + in both representative sub-perennial and perennial settings was examined. As anticipated, the added benefit was larger in sub-perennial settings. However, the favourable net impact of the proposed hybrid schedule was also predicted in perennial settings. These results indicate that, regardless of year-on-year rainfall and transmission uncertainty, hybrid chemoprevention delivery will likely increase the effectiveness.
Finally, it was acknowledged that conducting implementation studies in real-world scenarios is crucial to translating model-driven insights into policy or recommendations. As discussed, results from seasonally-targeted IPTi trial in Senegal [8] and modelling results built on IPTi trial data from Ghana [10] indicated a substantially larger impact of seasonally-targeted SP over a decade ago. However, no follow-up investigation or implementation occurred. Data collection for formal implementation research is beyond the scope of this modelling study; nevertheless, it aimed to address some likely next steps. This work is intended to facilitate broader discussion to ensure that new chemoprevention strategies advance beyond theoretical analysis and contribute to reducing the malaria burden in practice. To do this, possible implementation designs [15] and potential determinants of implementation for the proposed HMC [16] were presented based on literature.
Although SP is a standard of care when given as PMC, data still needs to be generated to understand the effectiveness, feasibility including cycle coverage and potential mode of delivery for the seasonally-targeted dosing, and the costs of alternative delivery strategies to support resource-allocation decisions. Therefore, hybrid type 2 effectiveness-implementation research will likely be helpful. Safety and efficacy studies may not be required given what’s known about safety & efficacy of individual doses of SP. As such, each dose protects for a certain period of time (depending on the resistance context) and that severe adverse reactions (primarily Stevens-Johnson syndrome) are idiosyncratic rather than dose-dependent [38]. Hence, evaluating effectiveness, consolidating safety and understanding impact is important. Furthermore, collecting qualitative data for the contextual determinants for tailoring delivery strategies to local contexts will be crucial to understanding how to begin rolling out any new delivery schedule. However, alternative study designs may also be considered, such as hybrid type 1 effectiveness-implementation with a primary emphasis on assessing clinical effectiveness and modest refinement to record the secondary implementation research goals. Programme success will depend on the availability of funds to deploy additional staffing at EPI facility and on the ability of community health workers to deliver both age- and seasonally-targeted dosing. Notably, expanding any prevention strategy will require comparing the cost-effectiveness of alternative approaches [23]. However, uncertainty around delivery methods and cost-per-dose estimates for seasonally targeted dosing limited economic analyses of HMC strategies in this study. It will be valuable in future work to explore cost-effectiveness again as cost data and delivery plans become available from potential implementation studies.
As with all modelling studies, these results have several limitations. First, the predictions are based on a model of blood-stage parasite-clearing activity for SP [23]. However, pyrimethamine may include liver-stage action, and any differences in immunity acquisition, assuming alternative mode of drug action dominates have not been explored [23, 32]. Also, the model does not estimate additional secondary benefits of SP beyond antimalarial effect (such as on bacterial or fungal infections) [39], which will further increase the total effect of HMC and HMC +. Second, outputs were not estimated by gender, given limited data on chemoprevention disaggregated by gender exists. Nevertheless, since the PK/PD model assumptions are consistent with earlier studies [23, 32], the broad conclusions regarding the impact of HMC and HMC + expect is expected to hold. Third, SP was modelled as used in PMC, though implementation might prefer SP-AQ during high-risk periods. However, mixing PMC with SMC would require careful consideration of different drug schedules [1]. Thus, proposed hybrid delivery schedules with SP were assessed only as a first step. SP is a relatively inexpensive, single-dose drug available within EPI and is likely to have better adherence compared to a three-day schedule. Increasing SP use may increase pressure on resistance development and spread. Thus, genomic surveillance would be prudent. Fourth, since there is currently no quantitative definition of sub-perennial transmission setting [6], a conservative threshold below strictly seasonal (i.e. less than 60% in consecutive four-month period [2, 3] was considered to define a representative sub-perennial setting. As a potential next-step for informing pilot implementation studies, the distribution of malaria cases over months was modelled based on rainfall pattern in parts of Mozambique [5, 23], where several pilot PMC (IPTi + projects) studies have been conducted [22, 23, 40]. However, results may vary in settings with different transmission profiles, such as flatter or two shorter rainy seasons [4] rather than a single prolonged one [5], reinforcing the need for a more precise definition. Finally, an implementation strategy was explored, only to initiate the discussion around possible designs.