Nano-sized medicine boosts anti-cancer immune response, eradicates tumors in mice
Image courtesy Alexander Stegh
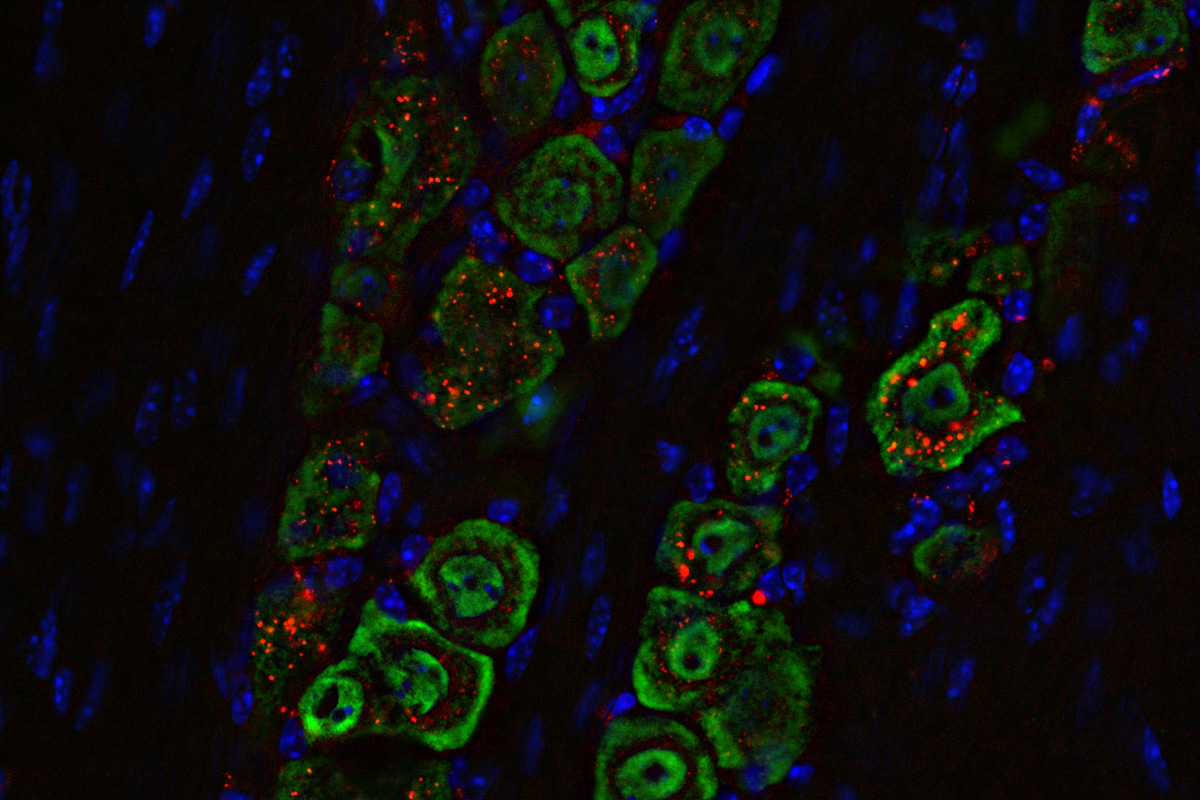
Researchers at WashU Medicine have developed a noninvasive medicine delivered through the nose that successfully eliminated deadly brain tumors in mice. The medicine is based on a spherical nucleic acid, a nanomaterial (labeled red) that travels along a nerve (green) from the nose to the brain, where it triggers an immune response to eliminate the tumor.
Researchers at Washington University School of Medicine in St. Louis, along with collaborators at Northwestern University, have developed a noninvasive approach to treat one of the most aggressive and deadly brain cancers. Their technology uses precisely engineered structures assembled from nano-size materials to deliver potent tumor-fighting medicine to the brain through nasal drops. The novel delivery method is less invasive than similar treatments in development and was shown in mice to effectively treat glioblastoma by boosting the brain’s immune response.
The findings were published this month in PNAS.
Glioblastoma tumors form from brain cells called astrocytes and are the most common kind of brain cancer, affecting roughly three in 100,000 people in the U.S. Glioblastoma generally progresses very quickly and is almost always fatal. There are no curative treatments for the disease, in part because delivering medicines to the brain remains extremely challenging
“We wanted to change this reality and develop a noninvasive treatment that activates the immune response to attack glioblastoma,” said Alexander H. Stegh, PhD, a professor and vice chair of research in the WashU Medicine Taylor Family Department of Neurosurgery and co-corresponding author of the study. Stegh also is research director of The Brain Tumor Center at Siteman Cancer Center, based at Barnes-Jewish Hospital and WashU Medicine. “With this research, we’ve shown that precisely engineered nanostructures, called spherical nucleic acids, can safely and effectively activate powerful immune pathways within the brain. This redefines how cancer immunotherapy can be achieved in otherwise difficult-to-access tumors.”
Cold tumors warmed with STING
Glioblastoma tumors are known as “cold tumors” because they do not induce the body’s natural immune response as do so-called “hot tumors” that are easier to treat with immunotherapies. Researchers have developed ways to spark an immune reaction against tumors by stimulating a pathway within cells called STING, which stands for stimulator of interferon genes. STING is triggered when a cell detects foreign DNA and activates the immune system to respond to the threat.
Past studies have shown that drugs activating STING in glioblastoma tumors can prime the body’s immune system to better fight the cancer. However, these agents break down quickly in the body and must be delivered directly into the tumor to work. Because repeated dosing is required for sustained benefit, relying on direct intratumoral administration requires highly invasive procedures.
“We really wanted to minimize patients having to go through that when they are already ill, and I thought that we could use the spherical nucleic acid platforms to deliver these drugs in a noninvasive way,” said Akanksha Mahajan, PhD, a postdoctoral research associate in Stegh’s lab and the first author on the study.
To overcome the problem, the Stegh team collaborated with co-corresponding author Chad A. Mirkin, PhD, director of the International Institute for Nanotechnology and the Rathmann Professor of Chemistry at Northwestern University, and his team. Mirkin invented spherical nucleic acids, a class of nanostructures that arrange DNA or RNA densely around a nanoparticle core, and he has shown that they have greater therapeutic potency compared to the standard delivery methods. The WashU Medicine and Northwestern researchers prepared a new class of spherical nucleic acids with gold cores studded with short snippets of DNA to trigger activation of the STING pathway in specific immune cells. To deliver these drugs to the brain, the team turned to the nose.
Intranasal therapy has been explored as a potential delivery method for medications targeting the brain, but no nanoscale therapies had yet been developed using this method to activate immune responses against brain cancers.
“This is the first time that it has been shown that we can increase immune cell activation in glioblastoma tumors when we deliver nanoscale therapeutics from the nose to the brain,” Mahajan said.
The team wanted to show that this approach could be used to deliver the medicine selectively to the brain, and that it would act on the appropriate cells once it got there. For the first objective, they used a molecular tag on the spherical nucleic acid that was visible under near-infrared light. They found that the nanomedicine, when delivered as droplets into the nasal passages of mice with glioblastoma, traveled along the path of the main nerve that connects facial muscles to the brain. The immune response evoked in the brain by the medicine was concentrated in the specific immune cells, especially those in the tumor itself, and triggered some helpful responses in the lymph nodes. The medicine did not spread to other parts of the body where it might cause unwanted side effects.
Examinations of immune cells in and near the tumor showed that the therapy successfully activated the STING pathway and armed the immune system to fight the tumor.
When applied in combination with drugs designed to help activate T lymphocytes, another type of immune cell, the new therapy eradicated the tumors with just one or two doses and induced long-term immunity against their recurrence. Taken together, the results were much better than those of current STING-activating immune therapies.
Stegh cautioned that firing up the STING pathway isn’t capable of curing glioblastomas without reinforcement from other therapeutic approaches. Turning on the STING pathway by itself isn’t enough to fight glioblastoma, because the tumor has many ways to block or shut down the immune response that STING is meant to activate. His team is looking to add capabilities to their nanostructure that activate other immune responses. This could allow physicians to double or triple the therapeutic targets all in a single therapy.
“This is an approach that offers hope for safer, more effective treatments for glioblastoma and potentially other immune treatment-resistant cancers, and it marks a critical step toward clinical application,” said Stegh.