In our nationwide observational study, we found a significantly higher risk of in-hospital mortality in haemoadsorption patients compared to a matched control group, suggesting that the widespread use of this technique in COVID-19 is not justified. Of note, ECMO support and CPR were also associated with increased mortality as independent variables in the logistic regression model. However, we emphasise that while ECMO and CPR are essential interventions that replace or restore life-sustaining functions, haemoadsorption is an adjunctive therapy whose potential benefits and risks to the patient must be carefully weighed. The use of haemoadsorption therefore requires more nuanced medical and ethical considerations for application in clinical practice.
Few small and non-confirmatory RCTs from Germany have evaluated the use of haemoadsorption with the CytoSorb® device in critically ill COVID-19 patients22,23,24. In patients with vasoplegic shock and renal replacement therapy (n = 49), 30-day mortality was not significantly improved by haemoadsorption (74% vs. 58% in the control group, p = 0.23). Neither effect on IL-6 levels, catecholamine requirement, nor other clinical endpoints was found in this study24. The CYCOV trial included COVID-19 patients supported with VV-ECMO (n = 34) and revealed a significantly higher 30-day mortality in the haemoadsorption group (76% vs. 18% in the control group, p = 0.0016), however, mortality was assessed only as a secondary endpoint23. Another RCT with COVID-19 patients failed to demonstrate a significant mortality benefit (n = 24; 28-day mortality 58% in the intervention group vs. 67% in the control group, p = 1.0). Of note, in this trial, increased IL-6 levels at or above 500 ng/L were among the inclusion criteria22. Despite a previous promising report of CytoSorb® therapy during VV-ECMO in COVID-19 patients (n = 8)18, no significant reduction of IL-6 plasma levels could be observed23,24.
In our cohort, in-hospital mortality was significantly higher in patients who received haemoadsorption compared to those treated without. These findings confirm concerns regarding the safety of uncritical use of this technique in clinical routine for severely ill COVID-19 patients. Recent studies challenge the relevance of hypercytokinemia in critical COVID-1914,15. A previous single-centre study shows significantly lower levels of IL-6, IL-8, and tumor necrosis factor (TNF) in patients with COVID-19-associated ARDS (n = 62) compared to septic shock patients with (n = 51) and without (n = 15) ARDS and a mixed picture when comparing COVID-19 to trauma (n = 62) and out-of-hospital cardiac arrest (n = 30)15. In a meta-analysis of multiple published cohorts, pooled mean IL-6 plasma levels for COVID-19 patients (n = 1,245) were significantly lower compared to other critical conditions, including chimeric antigen receptor (CAR)-T cell-induced cytokine release syndrome (100-fold difference, n = 72), hyperinflammatory ARDS (50-fold difference, n = 868), sepsis (30-fold difference, n = 5,320), and hypoinflammatory ARDS (5-fold difference, n = 1,899)14. A similar trend was observed for IL-8, TNF and other pro-inflammatory cytokines, while acute phase reactants were elevated, suggesting a hypoimmune state with virus-mediated tissue damage as the cause of critical COVID-1914. Based on these concerns and the findings from the above mentioned RCTs, the rationale and the safety for the routine use of haemoadsorption should be critically considered. We therefore suggest limiting the use of haemoadsorption in COVID-19 to carefully designed clinical trials.
Excessive release of cytokines is a hallmark of sepsis and ARDS independent from COVID-1914. Recent randomised evidence suggested that haemoadsorption may be useful as a therapeutic approach in sepsis and septic shock31. Based on the growing body of literature1, we hypothesised that a septic shock may have been the indication for haemoadsorption in our study population rather than critical COVID-19. To account for the interdependency of haemoadsorption and septic shock, we introduced interaction variables in regression modelling. The results confirmed that there was no survival benefit from haemoadsorption in either the overall group or in patients with septic shock. Similarly, a retrospective propensity score matched analysis of septic patients with hypercytokinemia (n = 143; mean initial IL-6 levels of 58,000–60,000 ng/L) showed neither difference in IL-6 reduction, haemodynamic stabilisation, nor mortality for patients treated with haemoadsorption32. These findings suggest that the use of haemoadsorption requires more stringent patient selection criteria and is not necessarily justified for septic shock patients with COVID-193,5.
Our granular analysis delineates several critical factors related to mortality among patients receiving haemoadsorption that should be considered and further assessed in clinical trials. Advanced age and poor health, as assessed by the Elixhauser score, pose a high risk of death. Haemoadsorption did not provide a survival benefit for critically ill COVID-19 patients who required ECMO, CPR and/or dialysis. Of note, more than half of the CPR patients in our haemoadsorption cohort (54.3%, 108/199) received this treatment after IHCA. Previously, the use of haemoadsorption showed no benefits after extracorporeal CPR in a single-centre RCT (n = 41) and was associated with higher mortality for out-of-hospital cardiac arrest patients (n = 72) in a propensity-matched single-centre registry study33,34. Due to inherent limitations, we cannot determine if the haemoadsorption treatment was related to CPR but nevertheless, our data do not suggest a positive impact of the haemoadsorption therapy on the mortality of COVID-19 patients at any time point after IHCA. While male sex is known to have a higher risk of COVID-19-related ICU admission and death35,36, gender was matched and did not play a detrimental role in our study population. The deployment of haemoadsorption correlated with higher numbers of coagulopathies, cardiac arrhythmia, and CPR but with lower numbers of pulmonary embolism and stroke. At the same time, coagulation disorders were associated with an increased risk of death in the control group, which is consistent with numerous studies confirming the cardiovascular manifestations of COVID-19 and their poor prognosis for patients37. The COVID-19 anticoagulation guidelines in Germany have been adjusted several times during the pandemic to balance the risk of thrombosis and haemorrhage38. Within our study, the patient cohort experienced a spectrum of treatment regimens reflective of an iterative learning process in protocol development, which may have led to an elevated rate of cardiovascular mortality in the control group due to suboptimal anticoagulation targets. In contrast, haemoadsorption therapy was administered under consistent, manufacturer-recommended anticoagulation protocols, potentially attenuating the risk of fatal cardiovascular outcomes. Acute liver failure was detrimental to survival in both groups, although previous case studies on the use of haemoadsorption for this indication in non-COVID-19 patients were positive39,40. Further, our data confirm a significant risk for cardiac arrhythmia associated with haemoadsorption therapy, which has also been reported in the RCT with vasoplegic shock patients (12 events in n = 10, 47.6% of patients in the haemoadsorption group vs. 3 events in n = 3, 11.5% patients in the control group, p = 0.0115)24. Patients with malignant diseases had a significantly increased risk of death if they were treated with haemoadsorption, which calls for particular caution. Even though a causality cannot be established with the current evidence, the risk factors derived in our study, among others, must be closely monitored and reported in future studies involving haemoadsorption.
Early initiation of haemoadsorption has been suggested for the treatment of septic shock patients19. In the retrospective CTC registry-based study (n = 100), early (≤ 87 h) use of haemoadsorption was associated with a reduction of the duration of mechanical ventilation (7 [2–26] days in the early treatment group vs. 17 [7–37] days in the late treatment group, p = 0.02), less days of ECMO support (13 [8–24] vs. 29 [14–38] days, p = 0.021), and shorter ICU stays (17 [10–40] vs. 36 [19–55] days, p = 0.002)20. However, no significant reduction of 90-day mortality was observed in the early treatment group (18%) compared to the late therapy start (34%)20, and the study suffered from serious methodological shortcomings, most importantly the absence of a control group21. We did not observe a significant impact on survival by early versus late therapy initiation in the multiple logistic regression model with a spline function controlling for therapy timing. Of note, our haemoadsorption group cannot be directly compared with the CTC patients, as our dataset does neither include laboratory values, respiratory parameters, nor the sequential organ failure assessment (SOFA) score, which are not included in the DESTATIS datasets. Nevertheless, 90-day mortality was remarkably lower among the retrospectively selected haemoadsorption patients on VV-ECMO support from the CTC registry (26%) than the in-hospital mortality in German ICUs (n = 357, 81% of all 443 VV-ECMO patients treated with haemoadsorption), suggesting a highly selected patient cohort in the CTC registry limiting the generalisability of the results. Still, the timing of COVID-19 treatment is crucial for immunomodulatory approaches like e.g. the widely accepted anti-IL-6 antibody tocilizumab41, and needs to be carefully evaluated also for haemoadsorption.
COVID-19 was shown to manipulate numerous biological pathways and cause a highly individual immune response42,43. Attempts to characterise the specificity of the CytoSorb® haemoadsorption device in septic and cardio-operative patients showed very different specificity profiles and demonstrated in some patients the elimination of apolipoprotein A1, serotransferrin, α-1-antitrypsin, immunoglobulins, and other proteins that are key in numerous biological pathways44. Many of these proteins are not quantified in routine clinical practice, making it difficult to understand their role for patient outcomes. It is therefore reasonable to assume that the non-specific and still undefined removal of biomolecules from the blood may have adverse effects that could reduce or eliminate the benefits of haemoadsorption in some patient groups. For example, longitudinal biomarker profiling of ECMO patients with and without haemoadsorption (n = 22) revealed a reduction of IL-10, that was speculated to exacerbate COVID-19-induced organ damage8. Further, clinicians must consider that haemoadsorption has been reported to adsorb various drugs and their active intermediates in vitro and in vivo9. Given the advanced age and poor health of non-surviving haemoadsorption patients, the removal of critical drugs such as immunosuppressants, antiepileptics, antiinfectives, and anticoagulants from the patients’ blood can be fatal.
Limitations
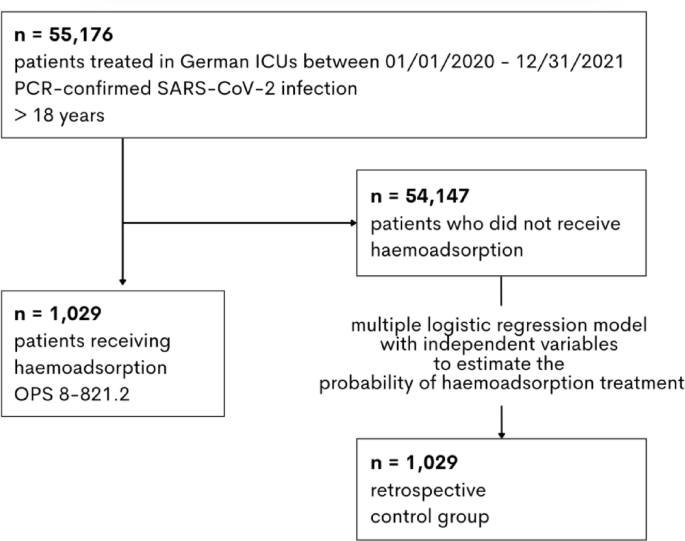
This observational study has limitations. The analyses were conducted with retrospective data, which were provided by the Federal Statistical Office. Due to the institutionally ensured anonymity, no conclusions can be drawn at individual patient level. Even if the sequence of events or treatment indications cannot be determined, we can confirm independent risk factors for mortality by regression modelling. We cannot provide laboratory data on inflammatory marker profiles or essential concomitant medication since they are in most cases not coded for reimbursement. Individual clinical parameters such as oxygen indices or the clinical presence of ARDS were not included in the matching process, as these variables are not coded for reimbursement and are therefore not available in the data set. Likewise, the reconstruction of clinical scoring systems (such as APACHE II or SOFA) using remuneration data alone is methodologically limited. To avoid misinterpretation suggesting the inclusion of clinical parameters (e.g. lab values or blood gas analyses), these metrics were not reported. This represents a limitation with regard to comparability with other studies that include detailed clinical parameters. Long-term survival analysis be conducted based on this dataset. In contrast, the Elixhauser Score a validated measure of disease severity derivable from remuneration records and is validated for hospitalised patients25,45. A possible coding-related bias in the Elixhauser score would affect all patient groups in our analysis equally. Furthermore, we assume that any facility-specific bias in our analysis is largely averaged out by the large number of hospitals (204 ICUs) covered in a short period of time. Our analysis does not provide indications and medical rationales for the use of haemoadsorption (e.g. myoglobin removal during rhabdomyolysis)46. Further, we cannot evaluate the physician’s assessment of the patient’s state. Specifically in our extremely vulnerable study group, the physician’s decision to use a costly and disputable therapeutic approach may have led to a bias in the selection of those patients who appeared to have the best chance of survival. Notably, the OPS codes for multiple haemoadsorption devices and no conclusion can be drawn regarding the differences in the devices from different manufacturers.