Diabetic foot ulcers (DFUs) are a major complication of DM, affecting up to 34% of diabetic patients. They contribute significantly to morbidity, healthcare costs, and lower limb amputations [1]. Current treatment often fails to achieve complete healing, particularly in chronic and ischemic ulcers [2]. Human umbilical cord-derived mesenchymal stromal cells (hUC-MSCs) are a promising therapeutic option due to their potent immunomodulatory, pro-angiogenic, and regenerative properties [4].
Central to the regenerative efficacy of hUC-MSCD is their secretion of key growth factors. Epidermal Growth Factor (EGF), a potent mitogen and a mainstay in wound healing, stimulates keratinocyte proliferation and migration—essential drivers of re-epithelialization—and also activates fibroblast proliferation and migration, facilitating granulation tissue formation and extracellular matrix remodeling [16]. Physiologically, serum levels of EGF in healthy individuals hover around ~ 30 pg/mL, and may range from 30 to 60 pg/mL in diabetics, although endogenous wound fluid concentrations are significantly lower, likely due to local depletion and enzymatic degradation. Restoring local EGF via hUC-MSCD-mediated secretion could potentiate epithelial closure and matrix deposition.
CXCL12 (SDF-1), another critical component in the hUC-MSCD secretome, plays a pivotal role in angiogenesis, recruitment of stem/progenitor cells, and immune modulation. CXCL12 signaling is essential for neovascularization and cellular recruitment during wound repair. Although systemic/homeostatic levels of CXCL12 have been recorded in the range of ~ 100–200 pg/mL, local levels in chronic wound environments are substantially lower, limiting effective healing [17]. Enhancing CXCL12 availability through hUC-MSCD may therefore rejuvenate vascular responses and cell trafficking to the wound.
Transforming Growth Factor-β1 (TGF-β1) is a multifunctional cytokine integral to all phases of wound healing—modulating inflammation, recruiting immune cells (especially macrophages), activating fibroblasts, synthesizing ECM and collagen, inducing angiogenesis (via VEGF), and regulating keratinocyte behavior and integrin expression for re-epithelialization. In DFUs, wound fluid TGF-β1 levels around 115 pg/mL have been associated with healing outcomes; levels above this threshold may predict closure within 12 weeks. Conversely, non-healing ulcers often show deficient or dysregulated TGF-β1 activity [18]. By supplying balanced TGF-β1 via hUC-MSCD, you may help re-establish the essential inflammatory-proliferative equilibrium needed for healing [19].
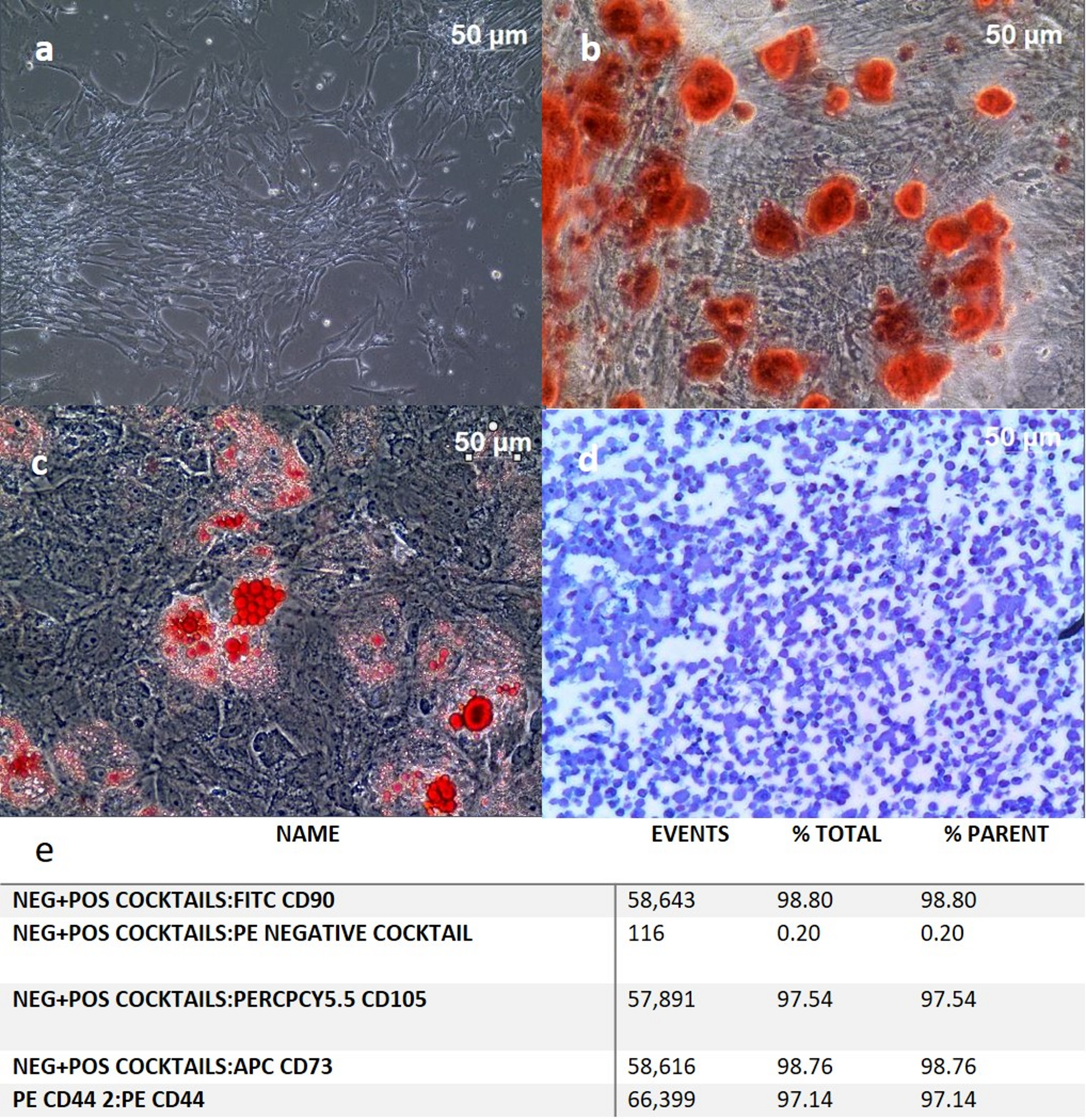
The term human umbilical cord mesenchymal stromal cell derivatives (hUC-MSCD) refers to bioactive components obtained from human umbilical cord mesenchymal stromal cells (UC-MSCs). These derivatives include exosomes, extracellular vesicles (EVs), and conditioned media, and they retain the therapeutic properties of UC-MSCs—most notably, immunomodulation and tissue regeneration—while being explored as cell-free therapies for a range of medical conditions [20].
Recent studies indicate that hUC-MSC derivatives such as conditioned medium, EVs, and exosomes may offer distinct advantages over direct use of UC-MSCs in therapeutic applications [20]. A key benefit is their reduced risk of tumorigenicity and immune rejection compared with other stromal cell types. UC-MSCs themselves exhibit low immunogenicity, making their derivatives particularly appealing for allogeneic transplantation [21]. Importantly, these derivatives preserve the regenerative capabilities of their parent cells while minimizing the ethical concerns associated with direct stem cell therapies.
Another significant advantage of hUC-MSCD lies in their paracrine activity, secreting bioactive molecules such as growth factors, cytokines, and EVs that promote tissue repair and modulate immune responses. These results validated the paracrine regenerative potential of the prepared hUC-MSCD. Studies have shown that hUC-MSC-derived exosomes display anti-inflammatory, pro-angiogenic, and anti-fibrotic properties, making them promising candidates for conditions including myocardial infarction, osteoarthritis, and neurodegenerative diseases [21]. Additionally, hUC-MSC-derived conditioned media have been shown to accelerate wound healing and reduce tissue damage by promoting cell migration and proliferation [22, 23]. These characteristics make hUC-MSCD a more accessible, scalable approach to regenerative medicine, avoiding the logistical and safety challenges associated with direct UC-MSC use—such as the need for extensive in-vitro expansion and concerns about long-term engraftment.
To date, only one clinical study has investigated allogeneic hUC-MSCs in diabetic foot ulcers (DFUs) in humans [9]. This trial examined both topical and intravenous administration of hUC-MSCs in DFU patients with peripheral arterial disease (PAD). Fourteen patients received treatment, which was found to be safe and effective, achieving >95% ulcer closure in all cases within 1.5 months [9]. Remarkably, no amputations or ulcer recurrences were reported after three years of follow-up.
In another study, stromal vascular fraction (SVF) derived from adipose-origin MSCs was tested in a phase I clinical trial involving 63 patients with type II diabetes and chronic non-healing DFUs [22]. Autologous adipose-derived SVF was delivered via local injections and was reported to be safe and effective: at 12 months, 50 patients achieved complete healing and four patients achieved ≥ 85% healing. Six patients died during follow-up and three underwent amputation.
Our study offers the unique advantage of not using MSCs from any source. Instead, we utilized the unfractionated conditioned media derived fromhUC-MSCs, the complete secretome, encompassing soluble growth factors, cytokines, and naturally released extracellular vesicles (including exosomes). In this phase I/II study, we characterized sterility, endotoxin absence, and the presence of key soluble mediators (EGF, CXCL12/SDF-1, and TGF-β1) at therapeutic concentrations, confirming biological activity of the preparation. We acknowledge that we did not separately isolate or analyze the extracellular vesicle and exosome fractions in this trial. Future preclinical and clinical studies will focus on detailed characterization of these subcomponents to further delineate their individual contributions to wound healing.
This product demonstrated excellent safety, with only mild short-term adverse events, and remarkable efficacy in accelerating DFU healing. The absence of ulcer recurrence during two years of follow-up further underscores its therapeutic promise. Moreover, the preparation can be stored as a ready-to-use product, without requiring additional cell culture or expansion prior to administration.
Although no mortality, amputations, re-hospitalizations, or cardiovascular events were observed during the 24-month follow-up, the small sample size limits the strength of conclusions regarding long-term outcomes, which should be rigorously evaluated in larger, controlled trials. While these results are encouraging, the small sample size limits the generalizability of our findings. Future work should focus on larger, placebo-controlled clinical trials to confirm efficacy, optimize dosing strategies, and further validate hUC-MSCD as a potential off-the-shelf biologic therapy for DFUs.