Introduction
Pain is defined as “an unpleasant sensory and emotional experience associated with, or resembling actual or potential tissue damage” []. In pediatric health care, pain is one of the most frequently reported concerns, and when inadequately managed, it may lead to long-term physical, psychological, and developmental consequences [,]. These risks underscore the urgent need for effective and safe pain management strategies tailored for children.
Current clinical recommendations emphasize multimodal approaches that integrate both pharmacological and nonpharmacological strategies to optimize outcomes in the pediatric population [,]. Pharmacologically, ibuprofen is the most extensively studied nonsteroidal anti-inflammatory drug and is widely recognized for its efficacy and safety in acute pediatric pain []. However, best practice not only achieves effective analgesia but also aims to minimize risks by reducing overreliance on pharmacological interventions and incorporating evidence-based nonpharmacological approaches [,].
In this context, socially assistive robots (SARs) have emerged as a promising nonpharmacological intervention for alleviating pain and mitigating emotional distress in pediatric health care settings [-]. Through features such as embodiment, personalization, empathy, and attentional distraction, SARs provide emotionally supportive interactions without requiring physical contact []. Evidence indicates that SARs can reduce procedural pain, anxiety, and distress while promoting positive affect and supporting postoperative recovery [-].
This potential is particularly relevant in hospital environments, where children frequently undergo painful and distressing medical procedures, such as injections, blood draws, surgeries, and cancer treatments [-]. Inadequately managed pain and distress in these settings may contribute to delayed recovery, prolonged hospitalization, long-term psychological sequelae, and reduced treatment adherence []. Compared with outpatients, hospitalized children are more often exposed to repeated and invasive procedures, making effective emotional support and pain management especially critical [].
Despite the growing interest, most existing systematic reviews of SARs have focused on outpatient applications, particularly in mental health or short-term procedural contexts, such as vaccinations and dental visits [,,,]. A few meta-analyses have examined SARs in clinical settings for outcomes such as anxiety [], pain and negative affect during needle-based interventions [], and psychological well-being []. Emotional responses are inherently subjective experiences [,]. However, previous meta-analyses included a blend of observer-rated and self-reported outcome measures. This study prioritized children’s self-reports, which are more accurately captured through their own perspective.
Furthermore, research on human-robot interaction highlights that the clinical implementation of SARs requires careful consideration of ethical dimensions, such as safety, privacy, and autonomy [,]. Ethical concerns also include children’s potential emotional overdependence, unintentional attachment, and reduced meaningful human interaction, which are especially salient for younger patients undergoing emotional and social development [,]. However, these dimensions have received limited systematic attention in pediatric care.
To address these gaps, this systematic review with meta-analysis synthesizes findings exclusively from randomized controlled trials (RCTs) that evaluated the effectiveness of SARs in reducing pain and emotional outcomes, including anxiety, fear, and distress, among pediatric patients in hospital settings. In addition, this study provides a comprehensive synthesis of intervention design and contextual factors for future RCTs, ultimately improving clinical outcomes and enhancing children’s hospital experiences.
Methods
Study Design
This review was prospectively registered in the PROSPERO (International Prospective Register of Systematic Reviews; CRD420251026751). This study followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 guidelines [] and the PRISMA-S (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Literature Search Extension) extension for literature searches (checklist provided in ) []. The search strategy was peer reviewed by a senior medical librarian before execution using the PRESS (Peer Review of Electronic Search Strategies) guidelines to ensure transparency, reproducibility, and methodological rigor []. Two reviewers independently conducted the study selection, risk of bias assessment, certainty of evidence appraisal, and data extraction. Discrepancies were resolved through discussions with a third reviewer and the corresponding author.
Eligibility Criteria
This review included RCTs that met the following eligibility criteria according to the PICO framework: (1) population (P): participants were children <19 years of age in hospital settings; studies focusing on children diagnosed with autism spectrum disorder were excluded, as previous research has already established the efficacy of SARs in this population []; (2) intervention (I): involved the use of SARs, excluding studies focused on rehabilitation, training, or surgical applications; (3) comparison (C): studies included control or alternative intervention; and (4) outcomes (O): the primary outcome was pain. Secondary outcomes were emotion-related responses.
Information Sources
A total of 8 electronic databases across 5 platforms were searched to identify relevant studies: PubMed (National Library of Medicine), MEDLINE (National Library of Medicine), Embase (Elsevier), Cochrane Library (Wiley), Scopus (Elsevier), IEEE Xplore Digital Library (IEEE Xplore), Health & Medical Collection (ProQuest), and ProQuest Dissertations & Theses A&I (ProQuest). To identify additional gray literature and unpublished studies, we searched the study registry ClinicalTrials.gov and manually screened conference proceedings from the Proceedings of the 2025 ACM/IEEE International Conference on Human-Robot Interaction. Both cited and citing references of relevant systematic reviews were examined by browsing their reference lists and using Google Scholar’s (Google LLC) citation function to identify additional eligible studies.
Search Strategy
An iterative search strategy was developed following the PRISMA-S extension for the transparent and reproducible reporting of literature searches. The strategy combined Medical Subject Headings, related terms, and free-text keywords using Boolean operators to optimize the sensitivity and specificity. Search concepts were informed by the PICO framework and included terms related to “hospitalization,” “child,” “social robot,” “pain,” “distress,” “emotion,” “anxiety,” “fear,” and “well-being.” The search syntax was subsequently adapted to each database’s indexing system. The initial search was conducted on May 6, 2025, and updated on October 7, 2025, by rerunning the searches. No language or publication date restrictions were applied. The details of the search strategies, including full line by line search strings, filters, parameters, search dates, and retrieval counts, are presented in .
Selection Process
All references were imported into EndNote (version 21; Clarivate), and the duplicates were automatically removed. Titles and abstracts were independently screened by 2 reviewers, followed by full-text assessments based on predefined eligibility criteria. The reasons for exclusion are documented in . The overall selection process is illustrated in the PRISMA flow diagram in the Results section.
A total of 1229 records were retrieved from 8 databases and 1 from citation searching. After removing 216 duplicates and screening titles or abstracts, 80 full texts were assessed. After 67 were excluded due to not meeting the criteria, 13 studies were included, with 7 providing sufficient data for meta-analysis.
Quality Assessment
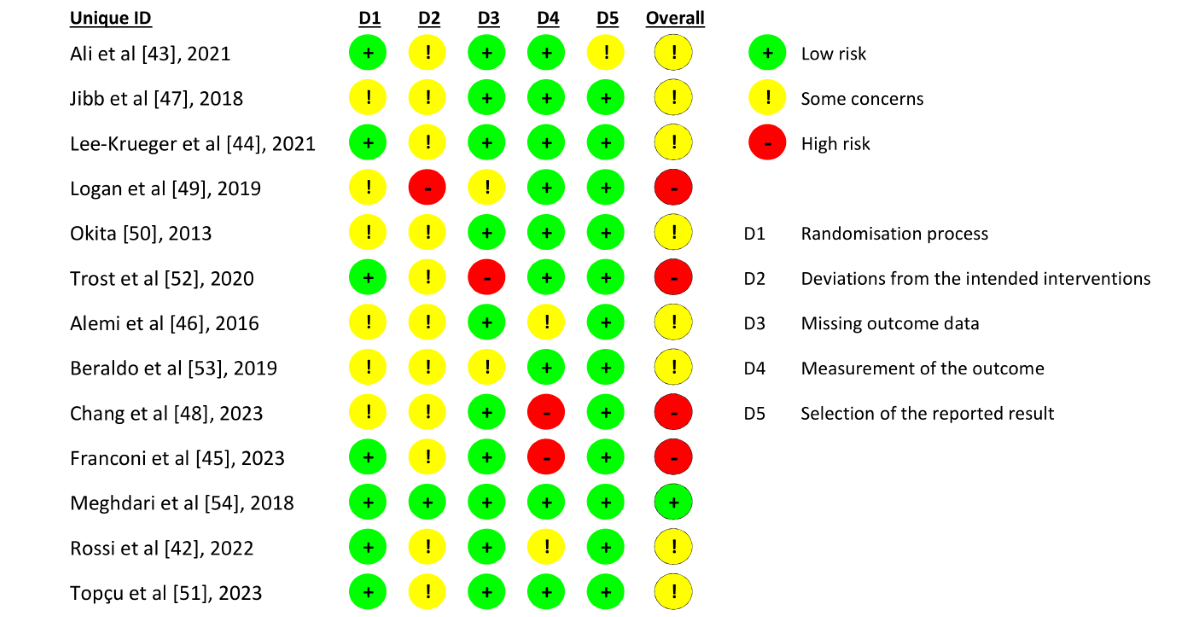
The methodological quality of the included RCTs was evaluated using the short version of the revised Cochrane Risk of Bias tool for randomized trials []. The risk of bias was assessed across 5 domains: randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results. Each domain was rated as “low risk,” “some concerns,” or “high risk” of bias, and an overall judgment was made.
Certainty of Evidence
The certainty of evidence for each outcome was assessed using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach []. Five domains were evaluated: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Outcomes were rated as “high,” “moderate,” “low,” or “very low” certainty of evidence. The ratings were generated using the GRADEpro Guideline Development Tool [].
Data Extraction and Synthesis
The data extraction included study characteristics such as authors, year of publication, country, study objectives, sample size, study population, participant age, setting, type of SARs, intervention details, comparator, measurement tools, and main findings. All the included studies contributed to the narrative synthesis. For the meta-analysis, only studies that provided sufficient numerical data were eligible for pooling, regardless of whether the outcome was primary (pain) or secondary (emotional responses). Where such data (eg, means, SDs, and sample sizes) were incomplete, we attempted to contact the original study authors to obtain additional information. Data synthesis was conducted in two parts: (1) narrative synthesis, summarizing key characteristics and findings of all included studies; and (2) meta-analysis, performed for outcomes with adequate quantitative data.
Data Analysis
Meta-analyses were conducted using R version 4.2.1 (R Project for Statistical Computing). Pooled effect sizes were estimated using a random-effects model to account for anticipated heterogeneity []. The outcomes included pain, anxiety, distress, and fear. For each outcome, differences in means with corresponding 95% CIs were calculated to accommodate variability across measurement scales. Subgroup analyses or meta-regression were planned in the presence of substantial heterogeneity. Given the limited number of studies, the Hartung-Knapp-Sidik-Jonkman method was applied to adjust the SEs []. Between-study heterogeneity was quantified using the inconsistency index (I²), between-study variance (τ²) and SD (τ), and 95% prediction intervals (PI) were reported to indicate the expected range of effects in future studies, except for outcomes with very few studies []. Forest plots were generated to visualize the pooled effect sizes. Funnel plots were constructed to assess the small-study effect. As recommended, Egger test was not performed for outcomes with fewer than 10 studies because of its low statistical power to detect true asymmetry [,].
Results
Literature Search
As illustrated in , a total of 1229 records were retrieved from 8 electronic databases (), with no additional records retrieved through other methods. After removing 216 duplicates, 1013 records remained for review. Title and abstract screening excluded 933 papers based on the predefined inclusion and exclusion criteria, resulting in 80 papers for full-text reviews. Of these, 67 were excluded because they did not meet the eligibility criteria (). Ultimately, 13 RCTs were included in this review. The details of the search strategies are presented in .
Characteristics of Included Studies
The characteristics of the 13 included RCTs are shown in . All studies were published between 2013 and 2023 and were conducted in 6 countries: Canada, the United States, Italy, Iran, Turkey, and Taiwan. A total of 619 participants were enrolled (intervention group: 301 and control group: 318), with individual study sample sizes ranging from 11 to 103. Participants were aged 2-19 years, most of whom were of school age, and all were in pediatric hospital settings due to acute illness, chronic disease, or surgical procedures. Additionally, the settings in which the interventions were implemented were diverse. Two trials were conducted in emergency departments [,], 2 in surgical wards and operating rooms [,], 2 in oncology units or hematology clinics [,], 3 in pediatric wards [-], 1 in a postanesthesia care unit [], 1 in a radiology department [], 1 in a hospice unit [], and 1 in a hospital-based game room [].
| Author (year), country | Objectives | Number of participants (IGb/CGc) | Study population | Age (years) | Setting | Measurements | Main results |
| Alemi et al (2016) [], Iran | Exploring the effect of SARsd as a therapy-assistive tool | 6/5 | Children with cancer receiving active therapy | 7-12 | Oncology unit in the hospital | MASCe, CDIf, and CIAg | Improved anxiety, anger, and depression with emotional support. |
| Ali et al (2021) [], Canada | Effect of SARs during the invasive procedure | 43/43 | Require intravenous insertion | 6-11 | Emergency department | FPS-Rh and OSBD-Ri | Reduced distress; none in pain. |
| Beraldo et al (2019) [], Italy | Potential of SARs during invasive medical procedures | 14/14 | Inpatients prepared for invasive procedures (eg, spinal tap) | 3-19 | Hospice unit in the hospital | Emotion questionnaire | Overall, reduced negative feelings, increased positive emotions. Most rated the experience positively. |
| Chang et al (2023) [], Taiwan | Impact of SARs-assisted digital storytelling of intravenous procedure | 26/26 | Inpatients with intravenous access | 5-10 | Pediatric general ward in the hospital | MYPASj | Reduced anxiety and improved therapeutic communication, emotions, and engagement. |
| Franconi et al (2023) [], Italy | Potential of SARs during the preoperative preparation | 30/30 | Preparing to undergo surgery | 2-14 | Pediatric surgical ward and operating room in the hospital | CEMSk | The intervention group showed significantly lower anxiety levels. |
| Jibb et al (2018) [], Canada | Impact of SARs during subcutaneous port access insertion | 19/21 | Children with cancer and a subcutaneous port underwent active therapy | 4-9 | Hematology clinic in a pediatric hospital | FPS-R, CFSl, and BAADSm | SARs were acceptable, but had no effect on pain or distress. |
| Lee-Krueger et al (2021) [], Canada | Effect of SARs support during intravenous induction | 45/58 | Required intravenous insertion before surgery | 4-12 | Operating room in a pediatric hospital | FPS-R and CFS | No significant differences in pain or fear across groups. |
| Logan et al (2019) [], United States | The feasibility and acceptability of SARs technology | 13/16 | Inpatient over 48 hours with cancer or surgery | 3-10 | General and hematology-oncology ward in a hospital | FPS-R, NRSn, FASo,PANAS-Cp, and STAI-Cq | Children exposed to SARs reported more positive emotion. SARs were mostly acceptable. |
| Meghdari et al (2018) [], Iran | Acceptability and involvement of SARs assistance | 7/7 | Children with cancer receiving active therapy | 5-12 | Game room in the hospital | TS-SFr and SAMs | Revealed high engagement and interest of pediatric patients with cancer with the SARs. |
| Okita (2013) [], United States | Potential of SARs companions and involvement with family | 9/9 | Hospitalized female children | 6-16 | General ward in a hospital | WBFPRSt and STAI-C | Significant reduction in pain and anxiety when children and parents engaged with SARs together. |
| Rossi et al (2022) [], Italy | Exploring the impact of SARs on stress before medical procedures | 36/37 | Waiting to access the medical office | 3-10 | Emergency department | Salivary cortisol levels and heart rate | Significant decrease in salivary cortisol levels and heart rate. The effect was stronger in girls. |
| Topçu et al (2023) [], Turkey | Effect of SARs on the postoperative recovery | 42/42 | Underwent day surgery | 5-10 | Postanesthesia care unit in a hospital | CSAu | Significant group differences in postoperative anxiety and mobilization time. |
| Trost et al (2020) [], United States | Impact of an empathic SARs during intravenous insertion | 11/10 | Required intravenous insertion before MRIv | 4-14 | Radiology department in a hospital | WBFPRS and CFS | Pain and fear significantly decreased over time. |
aRCT: randomized controlled trial.
bIG: intervention group.
cCG: control group.
dSAR: socially assistive robot.
eMASC: Multidimensional Anxiety Children Scale.
fCDI: Children’s Depression Inventory.
gCIA: Children’s Inventory of Anger.
hFPS-R: Faces Pain Scale-Revised.
iOSBD-R: Observed Scale of Behavioral Distress-Revised.
jMYPAS: Modified Yale Preoperative Anxiety Scale.
kCEMS: Children’s Emotional Manifestation Scale.
lCFS: Child Fear Scale.
mBAADS: Behavioral Approach-Avoidance Scale.
nNRS: Numeric Rating Scale.
oFAS: Facial Affective Scale.
pPANAS-C: Positive and Negative Affect Scales for Children.
qSTAI-C: State-Trait Anxiety Inventory for Children.
rTS-SF: Transportation Scale-Short Form.
sSAM: Self-Assessment Manikin Questionnaire.
tWBFPRS: Wong-Baker FACES Pain Rating Scale.
uCSA: children’s state anxiety.
vMRI: magnetic resonance imaging.
Design of SARs Interventions and Comparators
The included interventions varied in terms of timing, frequency, and technological features (). Six studies implemented SARs before or during invasive procedures [,,,,,], 4 addressed broader hospital experience contexts [,,,], 2 focused on preoperative care [] or postoperative care [], and 1 was conducted before a noninvasive procedure []. The intervention duration ranged from 3 to 40 minutes; 11 studies used a single session, while 2 adopted repeated sessions [,]. SARs primarily provide distraction, cognitive behavioral strategies, and emotional companionship. Technical difficulties were reported in 4 studies [,,,], mainly due to connectivity or hardware malfunctions, with rates ranging from 9% (4/46) to 60% (26/43).
| Author (year) | Type of SARs | Interventions | Comparators | Duration | Follow-up | Technical difficulties |
| Alemi et al (2016) [] | NAO | The hybrid-operated SARs engaged children through specific dialogue with a psychologist | Alternative intervention (only with a psychologist) | 5 min | 8 sessions | None reported |
| Ali et al (2021) [] | NAO | The SARs were programmed with self-introduction, breathing guidance, and dance during intravenous insertion | Standard care | 5-10 min | No | Occurred in 60% (26/43): connectivity, delays, tablet freezing, volume issues, shutdowns, or falls |
| Beraldo et al (2019) [] | Pepper | The hybrid operative SARs interacted with dialogue, gestures, games, and music during invasive procedures | Alternative intervention (Sanbot robot) | Not reported | No | None reported |
| Chang et al (2023) [] | Kebbi | Preprogrammed with digital storytelling during intravenous insertion | Standard care | 40 min | No | None reported |
| Franconi et al (2023) [] | NAO | Through hybrid operative programs of speech, singing, and play, and distracted attention before surgery | Standard care | Not reported | No | None reported |
| Jibb et al (2018) [] | NAO | SARs were preprogrammed with CBTb strategies such as deep breathing and encouragement during subcutaneous port insertion | Alternative intervention (active distraction with NAO) | 7-10 min | No | 35% (14/40): connection loss, phrase repetition |
| Lee-Krueger et al (2021) [] | NAO | The SARs were preprogrammed to guide deep breathing exercises before intravenous induction for surgery | Standard care | 5-20 min (mean 10 min) | No | None reported |
| Logan et al (2019) [] | Huggable bear | Teleoperation to interact with children through speech, games, and touch | Alternative intervention (plush teddy bear) | 9-40 min (mean 26 min) | No | 9% (4/46): wireless interference, delays, malfunctions, and speaker failure |
| Meghdari et al (2018) [] | Arash | Telling stories through preprogrammed dialogue, expression, and gesture | Alternative intervention (an audiobook with the same stories) | 3 min | No | None reported |
| Okita (2013) [] | Paro | Accompanied by mom and interacted with autonomous SARs through contact | Alternative intervention (alone with the SARs) | 30 min | No | None reported |
| Rossi et al (2022) [] | NAO | The hybrid SARs engaged children with songs, stories, jokes, and riddles before the medical procedure | Standard care | 15 min | No | Background noise or mispronunciation required teleoperation |
| Topçu et al (2023) [] | Macrobot | In postoperative recovery, autonomous SARs encouraged and accompanied children during mobilization | Alternative intervention (nurses) | 4-10 min | 3 sessions | None reported |
| Trost et al (2020) [] | MAKI | During intravenous insertion, the SARs provided empathetic responses | Standard care | Not reported | No | None reported |
aSAR: socially assistive robot.
bCBT: cognitive behavioral therapy.
Across the 13 included RCTs, 6 studies compared the SARs interventions with standard hospital care. The remaining 7 studies used diverse comparators, including psychologist-led therapy [], another robotic platform [], an alternative SARs-based distraction program [], a plush teddy bear [], audiobooks delivering the same narratives [], being alone with the SARs [], and nurse-led postoperative recovery []. These variations in comparator conditions illustrate the heterogeneity of approaches in contextualizing the role of SARs in pediatric care.
Nine types of SARs were used in the included studies (). Their physical appearances can be broadly categorized as humanoid (eg, NAO byAldebaran, Pepper bySoftBank, and Arash), animal-like (Huggable and Paro by National Institute of Advanced Industrial Science and Technology), or robot-like (Sanbot by Sanbot, Kebbi by Nuwa, MAKI, and Macrobot by Silverlit). Most SARs interacted with children using voice and gestures, and visual aids through camera input. Humanoid robots typically feature advanced functions, such as facial expression recognition and tactile feedback. The operational modes varied across autonomous, hybrid, and teleoperated systems. Cost information was available in only 2 studies: Arash (US $6000) [] and MAKI (US $2985) []. The price of Macrobot (US $27-$78) [] was obtained from commercial retail websites. For the other SARs, pricing information was obtained from the manufacturer’s specifications. Overall, 6 SARs were commercially available products, whereas Huggable and Arash were developed in research laboratories, and MAKI was custom-fabricated using 3D printing technology.
| SARs | Cost (US $) | Appearance | Interaction features | Specifications | Type of operation |
| Arash [] | 6000 | Humanoid (134 cm tall and 24 kg) | Voice, vision, facial expression, and gesture | Microphones, sensors, facial expression recognition, voice localization, camera, and screen | Preprogrammed automation |
| Huggable bear [] | Not reported | Bear-like | Voice and gestures | Microphones, a camera, and fluffy | Teleoperated |
| Kebbi [] | 600 | Robot-like (32 cm tall and 2.5 kg) | Voice, vision, and gesture | Microphones, camera, screen, and touch sensor | Preprogrammed automation |
| MAKI [] | 2985 | Robot-like (34 cm tall and 2 kg) | Voice | Microphones, speech recognition, text-to-speech, and lights | Teleoperated |
| Macrobot [] | 27-78 | Robot-like (20 cm tall and 0.25 kg) | Gestures and people following | Obstacle sensor, battery-powered, and wheel | Automation |
| NAO [-] | 7500-13,000 | Humanoid (57 cm tall and 5.5 kg) | Voice, vision, and gestures | Microphones, camera, LED, text-to-speech, and face detection | Hybrid |
| Paro [] | 6000 | Seal-like (57 cm length and 2.7 kg) | Body movements react to stroking and cuddling | Microphones, fluffy, and touch sensor | Automation |
| Pepper [] | 32,000-49,900 | Humanoid (120 cm tall and 28 kg) | Voice, vision, gestures, animations, and people detection | Microphones, cameras, LED, touch sensors, and tablet screen | Hybrid |
| Sanbot [] | 8500 | Robot-like (90 cm tall and 19 kg) | Voice, vision, gestures, people detection and following, and animations | Microphones, cameras, LED, touch sensors, screen, and laser projector | Hybrid |
aSAR: socially assistive robot.
Risk of Bias and GRADE Assessment
Eight studies were assessed as having some concerns regarding the overall risk of bias [-,,,,,], and 4 were assessed as having a high risk of bias [,,,]. The most frequent high-risk domains were deviations from the intended interventions (domain 2) and measurement of the outcome (domain 4; ). As the SARs intervention could not be blinded, some concerns were particularly identified in domain 2, where 1 trial [] was rated as high risk because its control group may have had an active role beyond that of passive control, potentially influencing the comparison with the intervention group. Two other studies were rated as high risk in domain 4 because the individuals assessing the outcomes also participated in the intervention, which may have introduced observer bias [,]. Additionally, 1 trial was rated as having a high risk of missing outcome data because it did not report 2 missing participants [].
According to the GRADE assessment, all outcomes were rated as moderate-certainty evidence (). Pain reduction showed moderate-certainty evidence when compared with both standard and alternative care. Anxiety and fear reduction were also rated as moderate, indicating potential benefits but inconclusive effects. Distress reduction was similarly rated as moderate, supported by a single trial. Overall, these outcomes are considered clinically important; however, the certainty of evidence was limited by the risk of bias and the small number of studies.
The risk of bias was evaluated across 5 domains. Most of the studies were identified as having some concerns, with deviations from the intended interventions (domain 2) being the most prevalent source of bias.
Narrative Synthesis
The outcomes of the 13 studies varied by domain (). For primary pain level measures in 6 studies, significant reductions were observed in 1 study [], whereas the other 5 [,,,,] reported no significant differences, reflecting mixed evidence regarding the analgesic benefits of SARs. As participant and personnel blinding were unfeasible in SARs interventions, 4 trials were rated with some concerns, and 2 were high-risk in reporting bias and comparator response bias. Secondary emotion-related outcomes were anxiety, fear, distress, emotional engagement, state positive and negative emotion, and stress level. Stress-related physiological outcomes were more consistent across 1 trial, which demonstrated significant decreases in both salivary cortisol and heart rate []. Anxiety outcomes showed clearer benefits, with 6 studies reporting significant reductions [,,,,,], while studies had some concerns or a high risk of bias due to observer bias. Three studies reported null effects of fear [,,]. Of the 2 studies [,], only 1 reported a significant reduction in distress []. For state emotions, SARs enhanced emotional engagement and positive emotions in 2 studies [,]. Additionally, 2 studies documented greater engagement with SARs and narrative immersion [,]. Detailed statistical findings of each study are presented in .
| Author (year) | Pain | Anxiety | Fear | Distress | Stress | Emotional engagement |
| Alemi et al (2016) [] | NAa | ↓b (P=.002) | NA | NA | NA | NA |
| Ali et al (2021) [] | NSc (P=.13) | NA | NA | ↓ (P=.047) | NA | NA |
| Beraldo et al (2019) [] | NA | ↓ (P=.047) | NS (P=.06) | NA | NA | NA |
| Chang et al (2023) [] | NA | ↓ (P<.05) | NA | NA | NA | ↑d (P<.05) |
| Franconi et al (2023) [] | NA | ↓ (P=.03) | NA | NA | NA | NA |
| Jibb et al (2018) [] | NS (P=.07) | NA | NA | NS (P=.06) | NA | NA |
| Lee-Krueger et al (2021) [] | NS (P=.98) | NA | NS (P=.33) | NA | NA | NA |
| Logan et al (2019) [] | NSe | NA | NA | NA | NA | NA |
| Meghdari et al (2018) [] | NA | NA | NA | NA | NA | ↑ (P<.03) |
| Okita (2013) [] | ↓ (P<.001) | ↓ (P<.01) | NA | NA | NA | NA |
| Rossi et al (2022) [] | NA | NA | NA | NA | ↓ (P<.01) | NA |
| Topçu et al (2023) [] | NA | ↓ (P=.005) | NA | NA | NA | NA |
| Trost et al (2020) [] | NS (P=.758) | NA | NS (P=.472) | NA | NA | NA |
aNA: outcome not assessed.
b↓: significant decrease.
cNS: nonsignificant.
d↑: significant increase.
eThe exact P value was not reported in the original study.
Meta-Analysis
Among the 13 included studies, 7 met the criteria for this meta-analysis, involving a total of 359 participants. Pain was the primary outcome, whereas anxiety, fear, and distress were secondary emotional responses (). All pooled estimates were calculated using the Hartung-Knapp-Sidik-Jonkman random-effects method, and PIs were displayed on the forest plots, except for outcomes with very few included studies, such as fear and distress. Funnel plots were generated for pain and anxiety to provide a visual assessment for small-study effect (). As the number of included studies was very limited (pain, n=5; anxiety, n=3; distress, n=2; and fear, n=2), no Egger tests were conducted [].
| Author (year) | Pain | Anxiety | Fear | Distress | ||||
| IGa | CGb | IG | CG | IG | CG | IG | CG | |
| Alemi et al (2016) [], mean (SD) | NAc | NA | 1.89 (0.20) | 2.38 (0.43) | NA | NA | NA | NA |
| Ali et al (2021) [], mean (SD) | 2.71 (2.96) | 3.74 (3.08) | NA | NA | NA | NA | 0.78 (1.32) | 1.49 (2.36) |
| Jibb et al (2018) [], mean (SD) | 1.00 (2.30) | 1.40 (3.00) | NA | NA | NA | NA | 1.60 (1.30) | 1.40 (0.80) |
| Lee-Krueger et al (2021) [], mean (SD) | 2.74 (2.96) | 2.76 (2.97) | NA | NA | 1.13 (1.02) | 1.16 (1.26) | NA | NA |
| Okita (2013) [], mean (SD) | 2.78 (1.92) | 5.13 (2.30) | 1.64 (0.31) | 2.81 (0.53) | NA | NA | NA | NA |
| Topçu et al (2023) [], mean (SD) | NA | NA | 2.74 (2.6) | 4.5 (2.96) | NA | NA | NA | NA |
| Trost et al (2020) [], mean (SD) | 1.55 (0.30) | 2.47 (0.40) | NA | NA | 1.80 (1.33) | 2.10 (0.76) | NA | NA |
aIG: intervention group.
bCG: control group.
cNA: outcome not assessed.
Pain
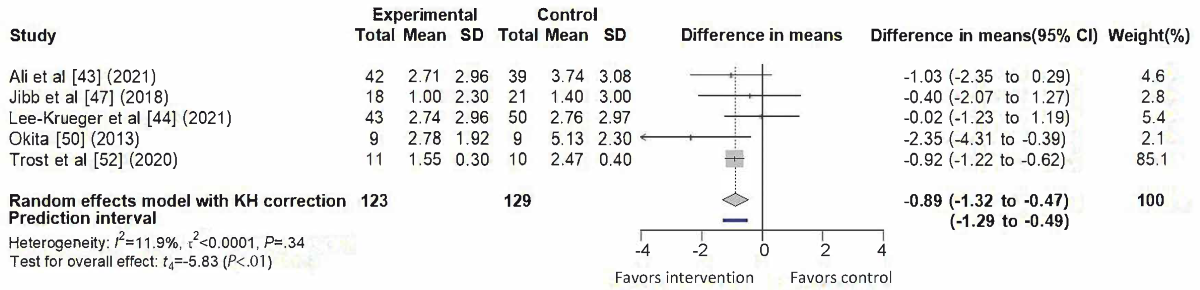
A total of 5 studies [,,,,] contributed data to the meta-analysis of pain outcomes, as illustrated in . The pooled analysis demonstrated a significant reduction favoring SARs interventions (difference in means=–0.89, 95% CI –1.32 to –0.47; 95% PI –1.29 to –0.49), with low heterogeneity (I²=11.9%, τ² < 0.0001, τ<0.01, P=.34). One study [] contributed the largest weight (85.1%), attributable to its smaller variance. The funnel plot showed slight asymmetry ().
Anxiety
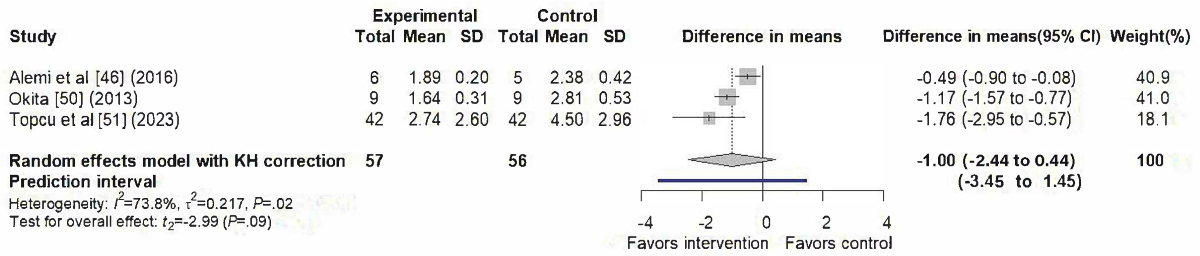
A total of 3 studies [,,] contributed to the meta-analysis of anxiety outcomes, as illustrated in . The random-effects model yielded a nonsignificant pooled effect (difference in means=–1.00, 95% CI –2.44 to 0.44; 95% PI –3.45 to 1.45), with substantial heterogeneity (I²=73.8%, τ²=0.2172, τ=0.466, P=.02). The funnel plot appeared symmetrical ().
Fear
A total of 2 studies [,] contributed to the meta-analysis of fear outcomes, as illustrated in the forest plot (). The pooled analysis showed no significant effect of SARs interventions (difference in means=–0.04, 95% CI –1.72 to 1.64), with no detected heterogeneity (I²=0%, τ²=0, P=.53).
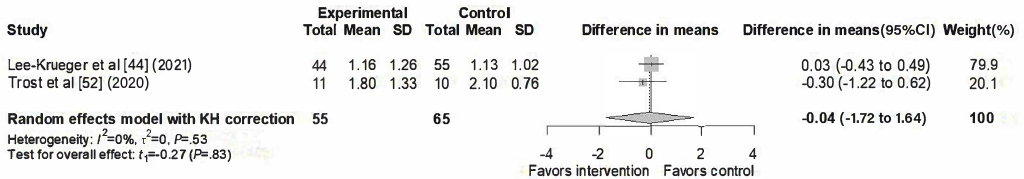
Distress
A total of 2 studies [,] were in the meta-analysis of distress outcomes, as illustrated in . The pooled analysis showed no significant effect of SARs interventions (difference in means=–0.23, 95% CI –6.00 to 5.54) with substantial heterogeneity (I²=65%, τ²=0.2693, τ=0.519, P=.09).
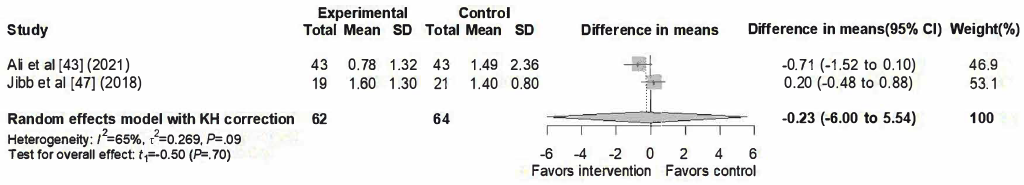
In summary, the meta-analysis provides evidence that SARs interventions may effectively reduce pain for children in the hospital. By contrast, the findings for anxiety, fear, and distress remain inconclusive due to nonsignificant pooled effects and considerable heterogeneity across studies.
Discussion
Principal Findings
This systematic review and meta-analysis synthesized evidence from 13 RCTs to evaluate the effectiveness of SARs in reducing pain and emotional outcomes, including anxiety, fear, and distress, among pediatric patients in hospital settings. Beyond the meta-analysis, our review conducted a comprehensive narrative analysis, integrating intervention characteristics and contextual factors to provide an understanding of real-world clinical implementation and future research design. Overall, the pooled analysis suggested that SARs interventions may offer beneficial effects for pain reduction, whereas their impact on emotional outcomes was not statistically significant. However, these findings should be interpreted with caution, given the presence of some concerns and high risks of bias in several domains, as well as the overall moderate certainty of evidence. Importantly, these results have practical relevance for health care providers and researchers, offering insights for future clinical implementation and study design aimed at adopting SARs as child-friendly and effective adjuncts in pediatric hospital care.
Pain
SARs interventions demonstrated a statistically significant reduction in children’s pain, providing moderate-certainty evidence that such interventions may help alleviate pain in hospital settings. Among the 5 studies synthesized, 1 trial [] was rated as high risk due to reporting bias and lack of blinding, while the others were rated as having some concerns. Notably, this high-risk study accounted for a large weight in the meta-analysis, suggesting that the pooled effect for pain may be disproportionately influenced by it and should therefore be interpreted with caution.
The PI was slightly narrower than, but consistent with, the effect of the CI. As prior studies [,], a narrower PI may indicate low between-study heterogeneity, which in this study could also reflect the large weighting of a single trial influencing the pooled estimate and reducing observed variability. This pattern suggests that similar beneficial effects may be observed under comparable conditions, but the limited evidence base warrants a conservative interpretation of these findings.
From a clinical perspective, these results imply that when intervention protocols, implementation settings, and participant characteristics are similar, clinicians may expect consistent and meaningful pain reduction with the use of SARs. In practice, SARs can provide distraction, emotional support, and engagement as adjuncts to standard pain management strategies. The combination of a statistically robust pooled effect and PI offers moderate yet credible evidence that SARs can reduce children’s pain perceptions during hospital-based procedures.
However, the duration of SARs interventions varied considerably across studies, revealing a lack of standardization in exposure time. Due to this variability, a dose-response relationship between intervention length and pain reduction could not be established. While short, single-session interventions may be well-suited for acute procedural pain, current evidence remains insufficient to confirm sustained benefits for children undergoing longer hospital stays. Collectively, these findings position SARs as promising, child-friendly adjuncts within multimodal pediatric pain management, though further methodologically rigorous and well-powered RCTs are needed to consolidate their clinical credibility, optimize implementation protocols, and determine long-term therapeutic potential.
Anxiety, Fear, and Distress
The emotional outcomes revealed a more complex and context-dependent pattern compared with the primary pain outcomes. Among the studies included in this review, SARs interventions appeared effective in reducing children’s anxiety when both self-reported and observer-rated measures were considered. However, the meta-analysis, which primarily focused on children’s self-reported anxiety scales, did not yield a statistically significant pooled effect. This divergence is likely attributable to differences in outcome measurement. Previous meta-analyses [-] reported significant reductions in anxiety, which typically combined observer-rated assessments with children’s self-reports, whereas our analysis distinguished between the two. This distinction reflects that anxiety, as an inherently subjective emotional experience, is best captured through the individual’s own perspective [,]. The nonsignificant result observed in our analysis aligns with prior evidence showing discrepancies between observer- and self-reported measures [], underscoring the need for further investigation into how these differing perspectives capture children’s emotional experiences. The overall moderate certainty of evidence reflects methodological limitations identified in the included trials, particularly the risk of bias from the nonblinded nature, inadequate statistical power, and reporting bias.
Furthermore, the CI reflects the average effect in this meta-analysis, while the wide PI illustrates the likely variation in true effects in future studies and clinical contexts [,]. The wide PI observed for anxiety suggests that the true effects of SARs may vary substantially across clinical contexts, indicating that while some settings may observe meaningful emotional benefits, others may experience null or even opposite effects. The statistical heterogeneity for anxiety and distress can be attributed to significant methodological and clinical context differences across the included trials. The studies varied widely in their clinical settings, study populations, intervention designs, and the specific features of SARs. Such variability likely reflects differences between included studies, rather than inconsistency in the underlying potential of SARs. This highlights the importance of contextual and implementation factors in shaping the emotional outcomes of SARs interventions. However, due to the limited number of studies, these findings should be interpreted with caution.
These contextual variations suggest that the effectiveness of SARs may be highly specific to a particular population, clinical context, or interaction mode. From a practical perspective, these findings emphasize the need for an approach grounded in real-world clinical contexts to ensure effective and meaningful integration of SARs into patient care. Overall, the evidence of SARs deployment for emotional support in pediatric hospital settings was limited, highlighting the need for more standardized trials to address these methodological and contextual variations.
Clinical and Practical Implications
The evidence from this review indicates that SARs represent an engaging and child-friendly adjunct for pain management in pediatric hospital settings. Our pooled results demonstrated a statistically significant reduction in pain, and the PI suggested that these benefits may be reproducible in similar clinical contexts. However, the current evidence for emotional outcomes remains limited and heterogeneous, emphasizing the need for caution in their implementation for psychosocial support.
The successful integration of SARs into clinical practice necessitates careful consideration of feasibility, ethical implications, and long-term sustainability. Clinically, SARs function primarily as assistants, supporting but not replacing human caregivers. Therefore, effective implementation requires comprehensive staff training in interaction protocols and hygiene management, alongside strong institutional support to ensure appropriate use and maximize clinical benefits. In addition, reliable technical support and regular maintenance are essential to sustain functionality, particularly in hospital settings that may have limited access to specialized technological personnel.
From an institutional perspective, performing a thorough cost-effectiveness analysis is essential. The initial acquisition costs of the SARs varied greatly and needed to be considered alongside the ongoing maintenance costs of hardware and software. A strategic evaluation of cost-effectiveness involving the adoption of innovative technologies, beginning with pilot studies to assess clinical feasibility before expanding to broader use, can further facilitate the full integration of SARs into health care settings.
Ethical Considerations
Ethical dimensions are critical for the implementation of SARs in pediatric hospital care, particularly regarding safety, privacy, and autonomy [,]. Only 4 of the 13 included studies addressed ethical considerations, primarily focusing on children’s physical and psychological safety [,,,]. The evidence currently offers limited insight into the broader ethical dimensions of human-robot interaction. Therefore, we expanded upon these critical ethical considerations.
Beyond safety, privacy is a crucial issue, requiring secure data storage, parental consent, and adherence to data protection standards [,,]. Psychological considerations and autonomy also warrant attention, while a few children may experience fear or negative experiences [,]. While SARs can provide comfort and support, some children may experience fear or discomfort [,,]. These risks intersect with the question of autonomy, particularly as children’s interactions with robots may influence their social and emotional development.
The automation level of SARs varied across included studies; notably, 11 trials used hybrid or operator-guided systems. Such approaches may represent the safest balance between technological novelty and patient safety in current clinical practice [,,,].
Strengths and Limitations
The primary strength of this review lies in its rigorous, systematic approach, coupled with the innovative integration of comprehensive contextual synthesis, cost-effectiveness, and ethical dimensions. The meta-analysis also allowed us to quantify and interpret the effect of SARs statistically. These contribute a framework for understanding SARs’ application relevant to real clinical practice.
However, several limitations should be acknowledged. The heterogeneity in methodological designs across included studies constrained the comparability of findings. The limited number of eligible trials presents a significant methodological constraint to performing subgroup analyses, particularly concerning statistical power. Although funnel plots were conducted to visually assess potential asymmetry, the small number of eligible trials constrained the reliable assessment of small-study effects (Egger test), as statistical power is limited with few studies []. Last, the moderate certainty of evidence underscores the need for greater methodological rigor in future research. In summary, these factors suggest that while the findings offer meaningful insights, they should be interpreted with appropriate caution and contextual awareness.
Future Research Directions
To address the risk of bias concerns identified in this review, future RCTs should adhere to rigorous methodological and reporting standards. Larger, well-designed, and adequately powered studies are warranted to reduce imprecision and enhance generalizability. As participant and personnel blinding are inherently unfeasible in SARs interventions, alternative strategies are suggested to minimize observer and response bias. These may include the use of blinded outcome assessors, standardized intervention protocols, and integrating objective indicators (eg, physiological parameters, objective behavioral indicators, speech emotion recognition, or facial expression recognition) to mitigate human influence during assessment.
As pain and emotions are inherently subjective experiences, self-reported measures remain the most direct indicators. However, combining validated self-report instruments with objective or observer-based assessments may provide a more comprehensive and balanced understanding. Transparent reporting of contextual and procedural factors will further facilitate comparability and reproducibility.
Moreover, research may expand beyond mitigating negative emotions to explore how SARs promote positive emotional responses and evaluate multisession interventions to determine sustained effects. Technological development is also crucial for improving system robustness, minimizing technical failures, and enhancing the usability of the operation. Notably, integrating ethical considerations, including child autonomy, privacy, and data protection, is essential for responsible future research.
Conclusion
This systematic review and meta-analysis suggest that SARs have potential as a valuable adjunct for pain management in pediatric hospital care. The observed reduction in pain across comparable clinical contexts indicates that SARs can provide consistent and clinically meaningful benefits when appropriately implemented. In contrast, the evidence for their effects on emotional outcomes remains ambiguous. The wide PI observed for anxiety suggests that the effects of SARs may vary substantially across clinical contexts, while some children may experience emotional benefits, others may show null or even opposite effects, highlighting the important role of contextual factors of SARs implementation. The overall concerns of risk of bias underscore the need for methodological rigor in future research to consolidate the evidence base.
At present, SARs can be regarded as a promising nonpharmacological tool for pain management. Their ethical and effective integration into pediatric practice requires adherence to clear principles that prioritize child-friendly care. Moving forward, research should combine technological innovation with psychosocial intervention design to evaluate the cumulative effects of multisession SARs interactions and to explore their potential to enhance positive emotions, engagement, and resilience. Through such evidence-driven and ethically grounded development, SARs may evolve into a vital component of child-centered digital health, fostering more positive and supportive health care experiences for children.
For significant contribution to the rigor and completeness of this review, this review’s authors gratefully acknowledge the studies’ authors for providing the original data for the meta-analysis. This study was partially funded by the Ministry of Science and Technology, Taiwan (NSTC 113-2410-H-182-011-MY2), and Chang Gung Medical Foundation (CMRPD1N0342). We used the GenAI (generative artificial intelligence) tool ChatGPT by OpenAI to assist with English language editing. We thank Dr Peter Pin-Sung Liu, Population Health Data Center, National Cheng Kung University, Tainan, Taiwan, for his assistance with statistical analyses and for providing valuable comments on the statistical methodology during the revision process. We also thank the Reference and Liaison Librarian for the College of Medicine, Ms Yi-hua Liu, for consulting on developing a detailed search strategy. All outputs were subsequently reviewed and revised by this study’s team.
All data analyzed in this study are included in the paper. Further details are available from the corresponding author upon reasonable request.
None declared.
Edited by A Mavragani, S Brini; submitted 07.May.2025; peer-reviewed by D Poddighe, S Ali; comments to author 12.Sep.2025; accepted 24.Oct.2025; published 26.Nov.2025.
©Fang Yu Hsu, Yun Hsuan Lee, Jia-Ling Tsai, Angela Shin-Yu Lien. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 26.Nov.2025.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research (ISSN 1438-8871), is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.