Carbon can exist in different forms — called allotropes — giving it completely different properties from color to shape to hardness. For example, in a diamond every carbon atom is bonded to four neighboring carbons, whereas in graphite, every carbon atom is bonded to three neighboring carbons. The newly-synthesized molecule is made up of 48 carbon atoms in an alternating single/triple bond pattern. Named cyclo[48]carbon, it is stable enough for spectroscopic characterization in solution at room temperature.
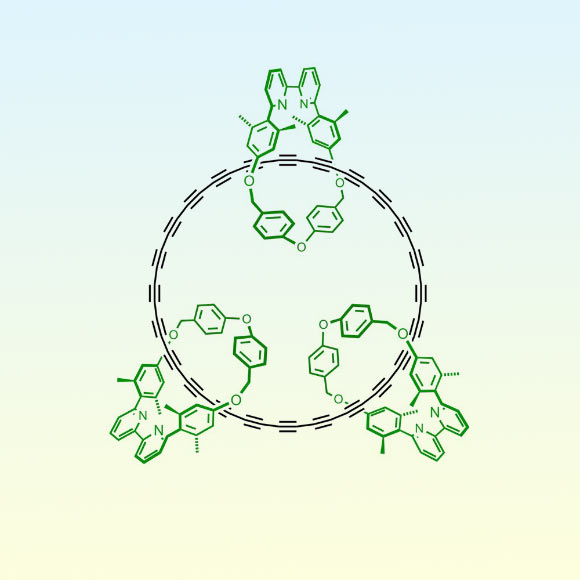
Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.
Dr. Yueze Gao from the University of Oxford and colleagues synthesized the cyclo[48]carbon molecule as a [4]catenane, i.e. with the C48 ring threaded through three other macrocycles.
These threaded macrocycles increase the stability of C48 by preventing access to the protected cyclocarbon.
Previously, molecular rings consisting purely of carbon atoms have only been studied in the gas phase or at very low temperatures (4 to 10 K).
According to the team, cyclo[48]carbon is stable in solution at 293 K (20 degrees Celsius).
This has been achieved by using threaded macrocycles, choosing a large cyclocarbon with a low level of strain, and developing mild reaction conditions for the unmasking step in the reaction (where a precursor molecule is transformed into the final product).
“Achieving stable cyclocarbons in a vial at ambient conditions is a fundamental step,” Dr. Gao said.
“This will make it easier to study their reactivity and properties under normal laboratory conditions.”
The researchers then used mass spectrometry, NMR, UV-visible and Raman spectroscopy to characterize the cyclocarbon catenane.
The observation of a single intense 13C NMR resonance for all 48 sp1 carbon atoms indicates that all of the carbons are in equivalent environments, which provides strong evidence for the cyclocarbon catenane structure.
“This achievement marks the culmination of a long endeavor to synthesize cyclocarbon catenanes, based on the hope that they might be stable enough to study at room temperature,” Professor Andersen said.
The team’s work was published in the journal Science.
_____
Yueze Gao et al. 2025. Solution-phase stabilization of a cyclocarbon by catenane formation. Science 389 (6761): 708-710; doi: 10.1126/science.ady6054