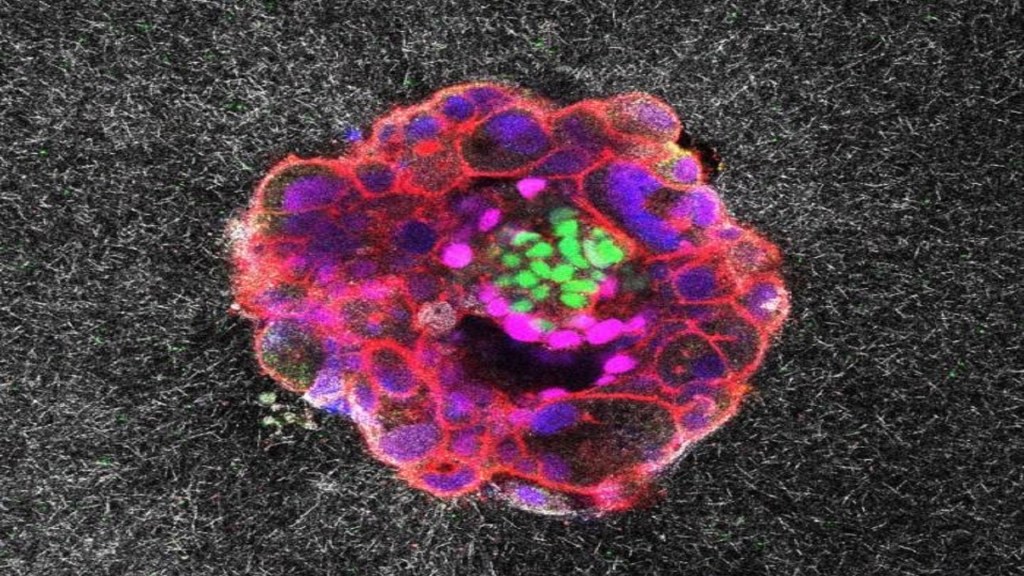
Researchers in Barcelona have, for the first time, recorded a human embryo embedding itself into the uterus in real time and in 3D, revealing the surprisingly forceful and invasive process that is essential for a successful pregnancy.
The breakthrough offers unprecedented insight into a critical stage of reproduction, long hidden from view, and could help tackle infertility linked to implantation failure.
Until now, studying implantation in humans was limited to still images taken at specific moments.
Embryos burrow into the uterus
Researchers at the Institute for Bioengineering of Catalonia (IBEC), in collaboration with the Reproductive Medicine Department at Dexeus Mujer–Hospital Universitari Dexeus, discovered that embryos exert significant mechanical force as they burrow into the uterine lining.
Samuel Ojosnegros, principal investigator, said the forces are necessary for the embryo to penetrate collagen-rich uterine tissue and fully integrate with the mother’s blood supply.
“We have observed that human embryos burrow into the uterus, exerting considerable force during the process. These forces are necessary because the embryos must be able to invade the uterine tissue, becoming completely integrated with it. It is a surprisingly invasive process,” he explained.
Co-first author Amélie Godeau added that embryos actively remodel their environment. They pull on the uterine matrix, moving and reorganizing it, and respond to external mechanical cues. The team hypothesizes that natural uterine contractions could influence implantation in vivo, highlighting the dynamic interaction between embryo and uterus.
To study implantation under controlled conditions, the researchers developed a lab platform mimicking the uterine environment.
The system uses a gel-based artificial matrix composed of collagen, a rigid protein also found in tendons and cartilage, combined with essential proteins for embryo development.
Uterus-embryo interaction captured
This setup allows real-time 3D fluorescence imaging and precise measurement of the forces applied by the embryos.
Experiments included both human and mouse embryos, revealing distinct implantation behaviors. While mouse embryos adhere to the uterine surface and are enveloped as the uterus folds around them, human embryos penetrate the tissue fully and grow radially from the inside out.
Anna Seriola, co-first author, said the platform enabled quantification of the “mechanical footprint” of these forces, providing insight into how embryos physically interact with the uterus.
The study relied on rigorously selected, ethically donated human embryos. Miquel Solé, director of the Laboratory of Cryopreservation at Dexeus Mujer, said careful selection ensured optimal conditions for the research.
The collaborative project also involved the Biomimetic Systems for Cell Engineering group at IBEC, the Barcelona Stem Cell Bank, the University of Barcelona, Tel Aviv University, CIBER, and IRB Barcelona.
The researchers note that video footage of the implantation process is available, giving scientists an unprecedented view of this critical stage.
By revealing the mechanical dynamics of implantation, the study could improve understanding of embryo quality, enhance assisted reproduction techniques, and reduce time to conception. Ojosnegros said, “Our work provides an unprecedented view of a process that has long been hidden from human eyes. Understanding the mechanics of implantation could transform reproductive medicine.”
The findings of the study has been published in Science Advances.