Threats relating to technology, disinformation, economic security and foreign interference are overshadowing traditional security concerns in Australians’ minds, according to data released by the Australian National University National Security College.
More than 12,000 people were asked across two surveys, in November last year and July this year, to rate the seriousness of 15 potential threats over the next decade.
Combining the categories of “major” and “moderate” the five most serious concerns were rated in July 2025 as:
-
the use of artificial intelligence to attack Australian people and businesses (77%)
-
a severe economic crisis (75%)
-
disruption to critical supplies due to a crisis overseas (74%)
-
the deliberate spread of false information to mislead the Australian public and harm their interests (73%), and
-
a foreign country interfering in Australia’s politics, government, economy or society (72%).
Climate change rated sixth (67%), although a high proportion of people (38%) rated it as a “major” threat. This was second only to threats relating to AI (40%).
The possible threat of Australia being involved in military conflict came in seventh (64%).
Anxiety about security issues is increasing. In July half the respondents agreed with the statement “I am worried about Australia’s national security”. This was an 8% rise between November 2024 and July.
Over that time, threat perceptions increased across all 15 possible threats that were asked about.
The table below shows the threat perceptions of about 6000 Australians in July.
Threat Perceptions July 2025
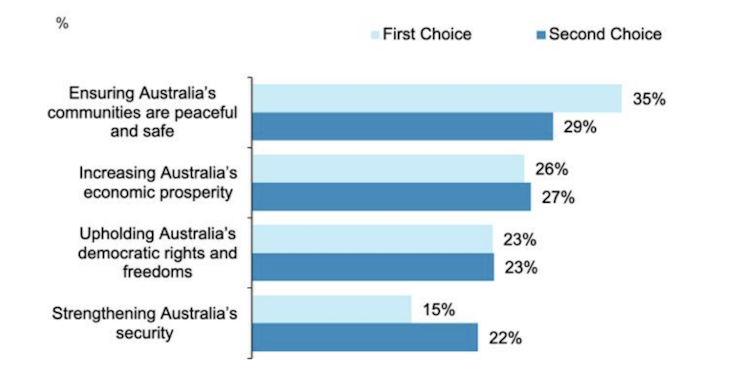
The November 2024 research also asked, from a list of four, what Australians want to nation to prioritise in the next five years.
The leading priority was safe and peaceful communities, nominated by 35%. When second preferences are included, this rises to 64%.
This priority ranked top across a wide range of demographics, including age, gender, cultural background, education , income and location.
The survey found three other national priorities rated in this order:
.. increasing Australia’s economic prosperity (26%)
.. upholding Australia’s democratic rights and freedoms (23%)
.. strengthening Australia’s security (15%).
The research also included more than 300 interviews across Australia.
The consultations found national security was “consistently framed as being about the peaceful continuity of everyday life”.
National priority for the next 5 years (%)
Australian National University
NSC head Professor Rory Medcalf said: “On the one hand, Australians know what they want to protect, especially in terms of peace, safety, community, democracy and prosperity, On the other hand, they recognise that a complex set of rapidly emerging threats can put these cherished priorities at risk.”
The full research results will be released early next year.
The ANU National Security College is a joint initiative of the federal government and the university.
The College undertook the community consultations as an independent research initiative.
