Our study reported a high rate of patients developing new pituitary deficiencies, up to 37.1% of 70 patients with a median follow-up of 20.7 months. These deficiencies could occur within the first six months following the end of radiotherapy. Few prospective studies have focused on pituitary deficiencies after irradiation and all of them used photons. Despite a short follow-up compared to retrospective studies, our cohort is one of the largest among prospective studies and the only one including multiple histologies. Similar to other studies, we report a high frequency of HP deficiencies [1, 9].
Median age was 60 years in our cohort as opposed to 47 years on average in other studies. Women were more frequent than men, which was related to predominance of meningiomas in this series. Patients irradiated for nasopharyngeal carcinoma (NPC) had the highest risk with a mean prevalence of 0.68 [1]. Dose constraints to the pituitary gland and hypothalamus were left to physician’s appreciation. In monitored patients, most pituitary glands received a mean dose > 50 Gy which prevented identification of deterministic effects and thresholds. Indeed, a mean PG dose > 50 Gy has been identified as a risk factor for pituitary deficiency in multivariate analysis [10]. Similarly, a correlation between the dose to the pituitary and the occurrence of hormonal axis insufficiency could not be observed [1], possibly because testing was primarily conducted in at-risk patients. In this population of patients receiving > 50 Gy to their PG per selection on endocrine monitoring, mean HT dose was not a risk factor (p = 0.56) although Partoune et al.reported a risk progressively increasing without a clear threshold [11]. It has been described that the higher the dose to the pituitary, the shorter the latency of GH deficiency [7]. This effect has never been reported for the other axes. However, it could be envisaged that it exists to a lesser extent due to a lower radiosensitivity. Larger prospective studies with a wider range of doses and regular endocrine assessments would be necessary to clarify its importance. Kyriakakis et al. described dose thresholds for every axis, from 10 Gy for GH to 50 Gy for TSH [12] and Darzy et al. reported mostly isolated GH deficiencies below 30 Gy [7].
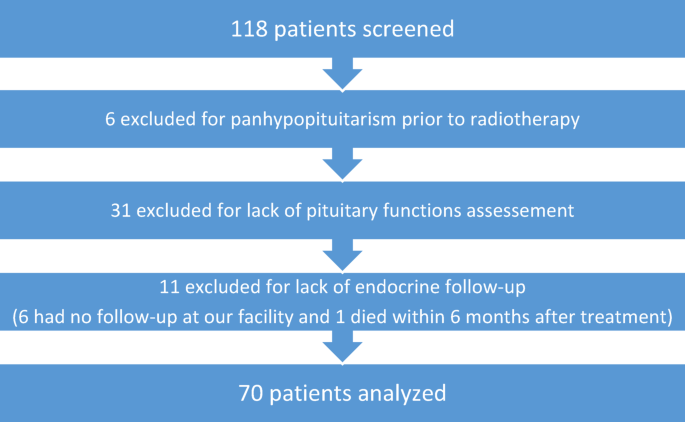
Our data of all five axes show that 37.1% of patients developed any new pituitary dysfunction within a median time of 14.1 months, including 17.1% within the first 6 months. Endocrine deficiencies have been reported as early as 3 months after radiotherapy, even at doses below 30 Gy [13]. We showed that pituitary deficiency occurred in at least one hormonal axis in 28.6% of cases and the number of surgical procedures before radiotherapy was an important risk factor; suggesting that it is critical to assess follow up levels with respect to baseline levels. Interestingly, among the 20 patients with deficits before radiotherapy, there was a trend toward cumulative or progressive pituitary dysfunction in patients with pre-existing impairment, consistent with a possible additive effect of surgery and irradiation. Moreover, repeated surgical interventions may increase the risk of damage to the pituitary gland or stalk, compromise vascular supply, or lead to scarring and adhesions that exacerbate radiation effects. This cumulative burden may explain the higher frequency of new deficits observed in patients with multiple surgeries [14]. Finally, GH dysfunction was 9.7% within 27.1 months, lactotroph dysfunction 35% within 13.5 months, gonadotroph 23.2% within 24.5 months, ACTH 3.2% with 27.0 months and TSH 7.8% within 21.8 months. In a recent meta-analysis, mean prevalence of endocrine insufficiency of 1 axis was 19%, 2 axes 22%, 3 axes 5% and panhypopituitarism 17% [1], of overall similar magnitude as in our cohort. Mean prevalence of GH deficiency was 40%, of higher magnitude than in our series, prolactin 22% of slightly lower magnitude, gonadotropin 20% of similar magnitude, while ACTH 16% and TSH 16% of higher magnitude than in our series [1]. A significant correlation is indeed observed between any endocrine insufficiency and follow-up time [1] and could explain the slight differences. Pituitary axes show different radiosensitivity in our series and the literature [1] and long term data are warranted for more personalized replacement therapies [7, 14, 15] and to promote long-term follow-up as HP deficiencies frequently occur after some years [10]. Identified differences in sensitivity vary between the anterior pituitary axes, with the growth hormone axis being the most easily damaged by irradiation and the thyroid hormone axis the least sensitive. Pituitary gland protection and early detection of deficiencies need further investigations. Of note, the hypothalamus seemed to be more vulnerable to radiation dose compared to the pituitary gland, which warrants further systematic, standardized and long-term monitoring data to establish reliable normal tissue complication probability (NTCP) models for HP deficiencies [1].
The rationale for using protons over photons lies in their particular dose deposition. The superiority of proton therapy lies in their excellent distal dose fall off at the end of the Bragg peak with virtually no dose behind, and this is particularly interesting to spare substantial volumes of distant organs. However, for relatively superficial tumors on the CNS and HN, most commercial pencil beam scanning proton therapy machines use a minimal 100 MeV, which requires the use of a range shifter to reach tumors less than 7.5 cm below the skin. Such energy degraders widen the lateral penumbra [16]. In tumors abutting the pituitary gland or the optic chiasm, stereotactic irradiation was sometimes used as the boost component to yield the steepest gradient-generating technique. Proton therapy however significantly spares more distant organs and the brain itself, which can have significant advantages on cognition compared to photons. Hypothalamic-pituitary deficiencies after proton therapy were described in two papers [17, 18]. In these retrospective studies of 74 (Lamba et al.) and 103 patients (De Marzi et al.), patients were treated for meningioma, chordoma or chondrosarcoma with a median mean dose to pituitary gland of 51.4 Gy and 54 Gy. Lamba et al. reported a new deficiency rate of 20% for any axis, occurring at a median time of 0.9 to 2.7 years after radiation depending on the axis. No difference was found between protons and photons regarding pituitary deficiencies. De Marzi et al. reported a 44% rate for any axis but no information regarding the duration of the follow-up was available. We conducted a comprehensive analysis of endocrine deficiencies and found that new deficits occurred in 40% of patients undergoing radiotherapy within a median follow-up of 5.6 years [19], in line with a more recent study [1] and the time-effect relationship. We observed similar rates of pituitary dysfunction between patients treated with protons alone and those who received combined photon-proton therapy. This may reflect comparable radiation doses delivered to the hypothalamo-pituitary axis across modalities, particularly in anatomically complex cases where sparing was limited regardless of technique. Few studies reported hypothalamic-pituitary deficiencies after proton therapy but no difference was found between proton-based and photon-based irradiations of tumors close to the PG, suggesting similarly steep gradient gradients.
A limitation of our study could be the absence of systematic dynamic tests, which probably led to a significant underestimation of the rate of somatotrophic and corticotropic partial deficiencies. However, a recent study of 246 patients referred to endocrinologists after cranial radiotherapy showed ACTH deficit was rare, and never isolated. The authors suggested that it may not be necessary to carry out a dynamic test for ACTH if no other deficits are diagnosed [15]. Another issue is GH supplementation in adults, especially in the context of a tumor. It remains widely debated, which might be one of the reasons why these deficiencies are not optimally investigated in routine practice [20]. GH replacement seemed to improve well-being parameters in adults but there were safety concerns [20, 21] about whether GH replacement increases future cancer risk. It is difficult to identify factors that modulate cancer risk in adults and there is some evidence for in-vitro pro-neoplastic properties and increased serum concentrations of IGF-I that could independently contribute to worse tumor or mortality outcomes in at-risk populations. Similarly, radiation-induced gonadotropic deficits may be overestimated in the absence of correction of associated asymptomatic hyperprolactinemia. We found that BMI < 25 kg/m2 is associated with earlier pituitary deficiency. To our knowledge, this association has never been reported and currently lacks pathophysiological explanation. The interpretation of prolactinemia may be particularly complex in this oncology context. This can only emphasize the need for more systematic and long-term assessment of the HP axes in oncology adults [22]. However, we showed a relative lack of patient adherence to follow-up of long-term side effects, particularly if asymptomatic. A limitation of our study and many others lies in a selection bias toward monitoring endocrine deficiencies only in patients at high risk of deficiency. Some patients were lost to follow-up at our facility or did not want to continue endocrine monitoring. At 3 years, 14 patients were evaluable and achieved over 85% compliance. This is common in oncology and might be improved with better education of patients, radiation oncologists and family doctors to promote a long-term standardized follow-up of those patients, considering that blood testing is hardly invasive. To overcome sampling and quantification biases, we considered using the raw data of plasma hormone levels from the cohort to describe the effects of irradiation. This approach provides additional information and has never been described in the context of hypothalamic-pituitary deficiencies. Moreover, it could be a first step towards modeling radiation-induced hypothalamic-pituitary effects. We included patients with pituitary resection and pre-existing pituitary deficits in the analysis, as they are at high risk of further deterioration, as long as they did not have complete panhypopituitarism. Monitoring this subgroup provides valuable insight into the potential additive or progressive impact of radiotherapy on residual pituitary function, which reflects real-world clinical scenarios and underscores the importance of individualized long-term endocrine follow-up.
The fact that this series is performed in patients undergoing proton therapy should not be misunderstood as an investigation of proton-specific side effects. More than ever, comparative studies and randomized photons/protons trials are warranted. Additionally, endocrine management guidelines [8] customized to the adult oncology context would be helpful to better standardize endocrine monitoring, its duration, based on the tumor characteristics and dose level, and replacement therapies in routine care [11, 12].