Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease associated with a significantly increased risk of cardiovascular (CV) disease, including coronary artery disease (CAD) and heart failure (HF) [1]. Despite advancements in glycemic control therapies, reducing CV morbidity and mortality in T2DM patients remains a critical challenge [2]. Although microvascular complications are closely associated with CV morbidity, and glycemic control effectively reduces microvascular damage, to date, no trial has conclusively demonstrated that lowering HbA1c alone significantly reduces major adverse cardiovascular events (MACE) in patients with established microvascular or macrovascular disease, i.e., in the context of secondary prevention [3]. However, some therapies have shown cardioprotective effects, reducing MACE independently of glycemic control [4]. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) represent a breakthrough in the management of T2DM, with extensive cardiovascular outcome trials (CVOTs) showing reductions in CV mortality, HF hospitalizations, and renal disease progression [5,6,7,8,9].
Coronary flow reserve (CFR), a key marker of cardiovascular health, particularly in patients with type 2 diabetes mellitus (T2DM) [10], is a fundamental indicator of coronary circulation, reflecting the ability of coronary arteries to increase blood flow during stress compared to rest (CFR = myocardial blood flow [MBF] at stress/MBF at rest) [11]. In T2DM, CFR is frequently reduced due to microvascular dysfunction, [12, 13] which contributes to a higher risk of ischemic events [6, 14, 15]. A potential cause of microvascular dysfunction may be the loss of the cardio protective effect of epicardial adipose tissue (EAT) [16, 17]. In type 2 diabetes, this distinct brown adipose tissue depot has been reported to undergo morphological and functional alterations shifting toward white adipose tissue characteristics along with increased thickening and inflammation16. Increased inflammation of EAT, indicated by the whitening of brown adipose tissue, has been widely documented in the literature [18,19,20,21]. Chronic inflammation contributes to microvascular dysfunction, which is detectable through a reduction in CFR [22, 23]. Due to its close anatomical proximity to the heart and its potential to regain its anti-inflammatory molecule secreting activity, thus improving CFR, EAT is now recognized as an important, independent, and modifiable cardiovascular risk factor [24,25,26].
Another potential consequence of microvascular dysfunction is the reduction in myocardial mechano-energetic efficiency (MEEi) [27], a measure of cardiac performance, which assesses the heart’s efficiency in converting metabolic energy into mechanical work [28]. MEEi, which is typically reduced in T2DM, due to cardiac insulin resistance and altered substrate utilization [28], provides valuable insights into the functional impact of therapeutic interventions on myocardial efficiency [15, 29]. Preserving or improving MEEi is essential for maintaining cardiac energy efficiency in this high-risk population.
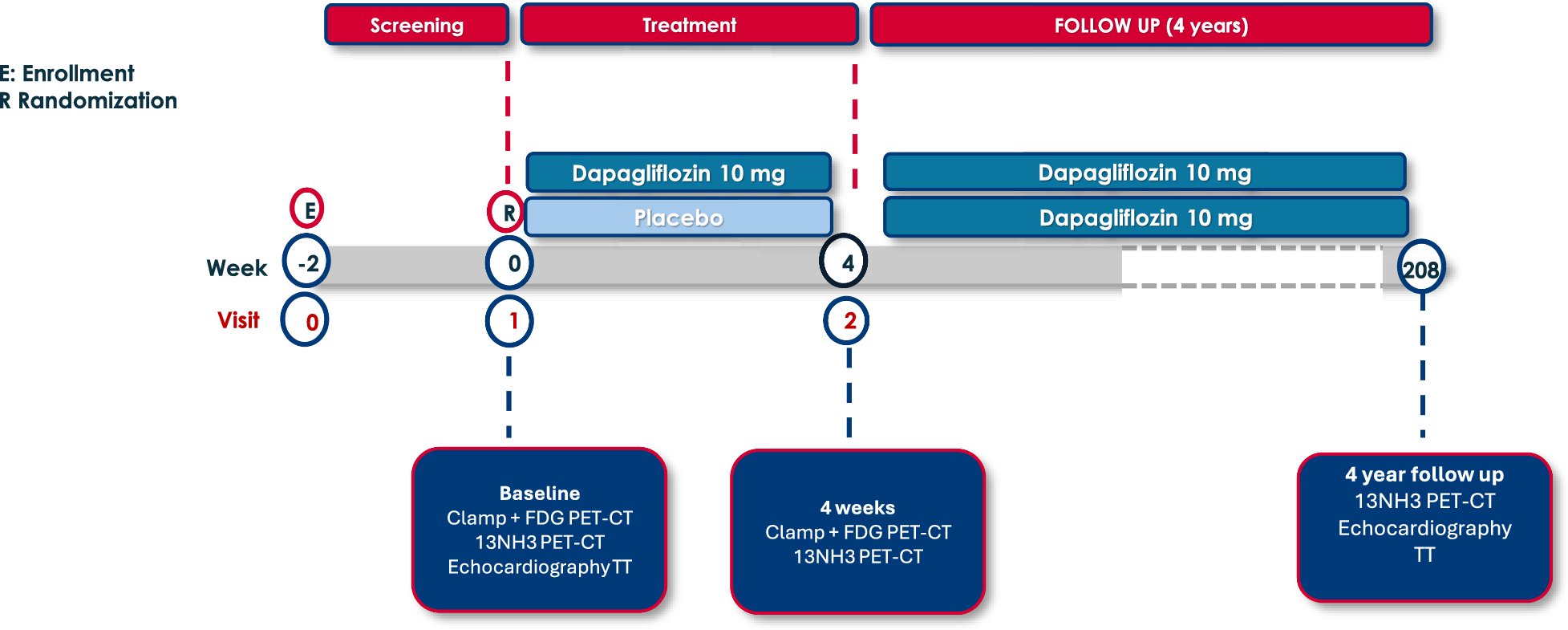
In the DAPAHEART trial, a recent single-center, 4-week, randomized, double-blind, controlled study (1:1 dapagliflozin 10 mg vs. placebo), we demonstrated that treatment with dapagliflozin induced a 30% increase in CFR in patients with T2DM and stable CAD [30, 31]. The improvement in CFR was associated with a selective 19% reduction in EAT thickness and glucose uptake, suggesting a restoration of the protective effect of this fat depot. Indeed, the reestablishment of the protective function of EAT may have effects on microvascular dysfunction, potentially accounting for the observed improvements in CFR , as previously mentioned [22, 23, 32].
However, whether these benefits are maintained over longer periods remains unexplored. This follow-up study aimed to address this knowledge gap by evaluating the long-term effects of dapagliflozin on CFR, EAT and MEEi over a 4-year period, providing insights into its sustained cardioprotective potential.