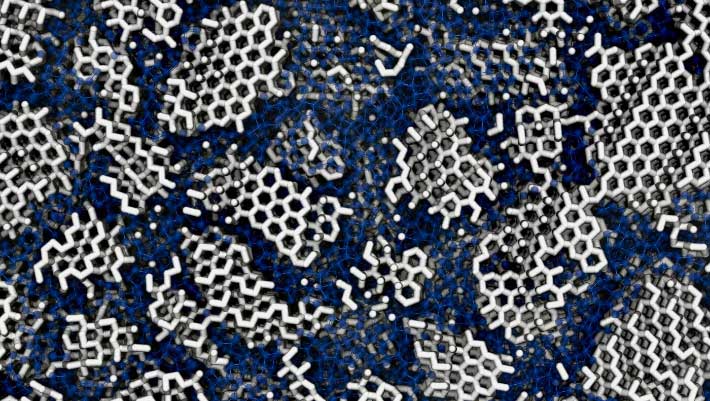
Low-density amorphous ice is one of the most common solid materials in the Universe and a key material for understanding the many famous anomalies of liquid water. Yet, despite its significance and its discovery nearly 90 years ago, its structure is debated. In a new study, researchers from University College London and the University of Cambridge found that computer simulations of low-density amorphous ice best matched measurements from previous experiments if the ice was not fully amorphous but contained tiny crystals — about 3 nm wide, slightly wider than a single strand of DNA — embedded within its disordered structures. In an experimental work, they also re-crystallized (i.e. warmed up) real samples of amorphous ice that had formed in different ways. They found that the final crystal structure varied depending on how the amorphous ice had originated. If the ice had been fully amorphous (fully disordered), the researchers concluded, it would not retain any imprint of its earlier form.
The structure of low-density amorphous ice: many tiny crystallites (white) are concealed in the amorphous material (blue). Image credit: Michael B. Davies, UCL & University of Cambridge.
“We now have a good idea of what the most common form of ice in the Universe looks like at an atomic level,” said Dr. Michael Davies, a researcher at University College London and the University of Cambridge.
“This is important as ice is involved in many cosmological processes, for instance in how planets form, how galaxies evolve, and how matter moves around the Universe.”
For their study, Dr Davies and colleagues used two computer models of water.
They froze these virtual ‘boxes’ of water molecules by cooling to minus 120 degrees Celsius (minus 184 degrees Fahrenheit) at different rates.
The different rates of cooling led to varying proportions of crystalline and amorphous ice.
The researchers found that ice that was up to 20% crystalline (and 80% amorphous) appeared to closely match the structure of low-density amorphous ice as found in X-ray diffraction studies (that is, where researchers fire X-rays at the ice and analyze how these rays are deflected).
Using another approach, they created large ‘boxes’ with many small ice crystals closely squeezed together.
The simulation then disordered the regions between the ice crystals reaching very similar structures compared to the first approach with 25% crystalline ice.
In additional experimental work, the scientists created real samples of low-density amorphous ice in a range of ways, from depositing water vapor on to an extremely cold surface (how ice forms on dust grains in interstellar clouds) to warming up what is known as high-density amorphous ice (ice that has been crushed at extremely cold temperatures).
They then gently heated these amorphous ices so they had the energy to form crystals.
They noticed differences in the ices’ structure depending on their origin — specifically, there was variation in the proportion of molecules stacked in a six-fold (hexagonal) arrangement.
This was indirect evidence that low-density amorphous ice contained crystals.
If it was fully disordered, the ice would not retain any memory of its earlier forms.
The findings raised many additional questions about the nature of amorphous ices — for instance, whether the size of crystals varied depending on how the amorphous ice formed, and whether a truly amorphous ice was possible.
“Water is the foundation of life but we still do not fully understand it,” said University of Cambridge’s Professor Angelos Michaelides.
“Amorphous ices may hold the key to explaining some of water’s many anomalies.”
“Ice is potentially a high-performance material in space,” Dr. Davies said.
“It could shield spacecraft from radiation or provide fuel in the form of hydrogen and oxygen.”
“So we need to know about its various forms and properties.”
The findings also have implications for one speculative theory about how life on Earth began.
According to this theory, known as Panspermia, the building blocks of life were carried here on an ice comet, with low-density amorphous ice the space shuttle material in which ingredients such as simple amino acids were transported.
“Our findings suggest this ice would be a less good transport material for these origin of life molecules,” Dr. Davies said.
“That is because a partly crystalline structure has less space in which these ingredients could become embedded.”
“The theory could still hold true, though, as there are amorphous regions in the ice where life’s building blocks could be trapped and stored.”
“Ice on Earth is a cosmological curiosity due to our warm temperatures,” said University College London’s Professor Christoph Salzmann.
“You can see its ordered nature in the symmetry of a snowflake.”
“Ice in the rest of the Universe has long been considered a snapshot of liquid water — that is, a disordered arrangement fixed in place. Our findings show this is not entirely true.”
“Our results also raise questions about amorphous materials in general.”
“These materials have important uses in much advanced technology.”
“For instance, glass fibers that transport data long distances need to be amorphous, or disordered, for their function.”
“If they do contain tiny crystals and we can remove them, this will improve their performance.”
A paper on the findings was published today in the journal Physical Review B.
_____
Michael Benedict Davies et al. 2025. Low-density amorphous ice contains crystalline ice grains. Phys. Rev. B 112, 024203; doi: 10.1103/PhysRevB.112.024203