The final week of December closes the year with a strong set of practice-shaping insights across GI oncology, spanning rectal, colorectal, pancreatic, esophageal, and biliary cancers. This week’s selection reflects how clinical decision-making continues to evolve at the intersection of intensified multimodality therapy, organ preservation strategies, liquid biopsy–guided precision oncology, and deeper biological understanding of treatment resistance and immune context.
From phase II data supporting non-operative management after total neoadjuvant therapy in rectal cancer, to refined patient selection for anti-EGFR rechallenge, ctDNA-driven trial interpretation, and real-world optimization of chemotherapy in older patients, these contributions highlight both the progress made in 2025 and the critical questions that remain unanswered. At the same time, advances in translational research—from organoid-based pancreatic cancer modeling to population-specific cancer genomics—underscore the growing importance of biology-driven frameworks to guide future trials and clinical practice.
Together, these ten posts capture a fitting end-of-year snapshot of GI oncology: rigorous, multidisciplinary, and increasingly personalized, with a clear focus on translating innovation into meaningful patient benefit.
Sebastian Adeberg, MD, PhD – Professor and Director, Clinic for Radiotherapy and Radiation Oncology, UKGM; Marburg Ion-Beam Therapy Centre (MIT), Germany
“Out now Total neoadjuvant therapy followed by non-operative management or surgery in stage II–III rectal cancer (NO-CUT): a multicentre, single-arm, phase 2 trial (The Lancet Group).
• 179 pMMR/MSS stage II–III rectal cancer patients treated with TNT
• 26% achieved clinical complete response → non-operative management
• 30-month distant relapse-free survival: 95% with organ preservation
• Low severe toxicity; no treatment-related deaths
• Post-TNT ctDNA showed predictive & prognostic value”
Davide Ciardiello, MD – Medical Oncologist, Division of Gastrointestinal and Neuroendocrine Tumors, IEO, Istituto Europeo di Oncologia, Milan, Italy
“Finding new effective strategies in chemo-refractory colorectal cancer is one of the greatest unmet needs.
The CAVE-2 GOIM clinical trial, an Italian, academic, phase II study, evaluated rechallenge with cetuximab alone or in combination with the anti–PD-L1 antibody avelumab in patients with chemo-refractory colorectal cancer without clonal RAS/BRAF alterations on liquid biopsy, assessed using the FoundationOne Liquid CDx extended genomic profiling assay.
The study showed that the addition of avelumab to cetuximab was associated with a numerical, but not statistically significant, improvement in progression-free survival and overall survival compared with single-agent cetuximab. This benefit appeared more pronounced in patients without liver metastases. The most interesting finding was that, regardless of treatment strategy, patients with so-called ‘ultra–wild-type’ tumors—lacking primary or secondary resistance alterations beyond RAS/BRAF—derived greater benefit in terms of objective response rate, progression-free survival, and overall survival.
These data support the implementation of liquid biopsy with multigene panels in clinical practice to optimally select patients who may benefit from anti-EGFR rechallenge strategies.”
Read full article here
Catherine Alix-Panabières, PhD – Full Professor of Oncology, Faculty of Medicine, University of Montpellier, France; Visiting Professor, University of Hamburg, Germany; Working Group Leader, European Liquid Biopsy Society (ELBS)
(Reposted from the European Alliance for Personalised Medicine – EAPM following Vision Europe 2030, Brussels)
“Voices from Vision Europe 2030: Catherine Alix-Panabières on Liquid Biopsy
At Vision Europe 2030, co-funded by the European Union and held in Brussels, Catherine Alix-Panabières shared powerful reflections on the past, present, and future of liquid biopsy in precision oncology.
Professor Panabières is a pioneer in the field, having co-coined the term liquid biopsy in 2010 alongside Professor Klaus Pantel, who also participated as a speaker at the conference. Drawing on more than 26 years of research, she highlights how collaboration, harmonisation, and shared European expertise are essential to accelerate clinical translation and move liquid biopsy into routine medical practice.
She also underlines the growing impact of the European Liquid Biopsy Society (ELBS), now uniting around 100 academic and private institutions across Europe, all working towards improved early cancer detection and more personalised treatments. As Working Group Leader for Dissemination and Education, she stresses the importance of equipping researchers and clinicians with best practices for clinical trials and real-world application.
Her message is clear and optimistic: strong European research networks are key to turning scientific innovation into tangible benefits for patients, shaping a more precise, collaborative, and patient-centred future in cancer care.”
Brice Chanez, MD, PhD – Medical Oncologist, Institut Paoli-Calmettes, Marseille, France
“Very proud to share our latest publication on toxicities associated to FFX in elderly with locally advanced and metastatic PDAC from the Institut Paoli Calmette DataBase:
Keys are
- Early evaluation and geriatric assessement
- Monitoring closely early side effects
- Malnutrition and sarcopenia are very common and a huge challenge
- Toxicities are manageables and survival similar to younger when toxicities are controlled !
Bravo Bérénice
Thanks to Girci To have funded that project”
Read full article here
Nelson Dusetti, PhD – Research Director, INSERM; Pancreatic Cancer & Translational Oncology; Co-founder, Predicting Med
“We are happy to announce that our project ‘PhenoPDAC’ has been selected for the 2025 Proof-of-Concept grant from FRAP Network – Pancreatic Cancer.
The project is led at the CRCM – Centre de Recherche en Cancérologie de Marseille in close interaction with the Institut Paoli-Calmettes, and aims to establish a proof-of-concept pipeline combining patient-derived organoid-CAF co-cultures, high-content live imaging and transcriptomic analyses, to better capture mechanisms of drug response and resistance in pancreatic cancer.
This work is carried out in close collaboration with Rémy Nicolle and his team at the CRI – Centre de Recherche sur l’Inflammation – INSERM U1149, who bring strong expertise in integrative bioinformatics, image-omics and predictive modeling, building on their previous contributions to the field.
We thank FRAP for their confidence and support, and for their structuring role in fostering high-quality collaborative research, enabling the development of shared tools and approaches for the pancreatic cancer research community”

Giovanni Marchegiani, MD, PhD – Academic Pancreas Surgeon, Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospital of Padova, Italy
“How to determine resectability of locally advanced pancreas cancer?!
Suitable target is a disease-free arterial or venous segment above and below the tumor involvement
‘Reconstructability’ is based on expertise and tumor biology”
Read full article here
Daisuke Kotani, MD, PhD – Medical Oncologist
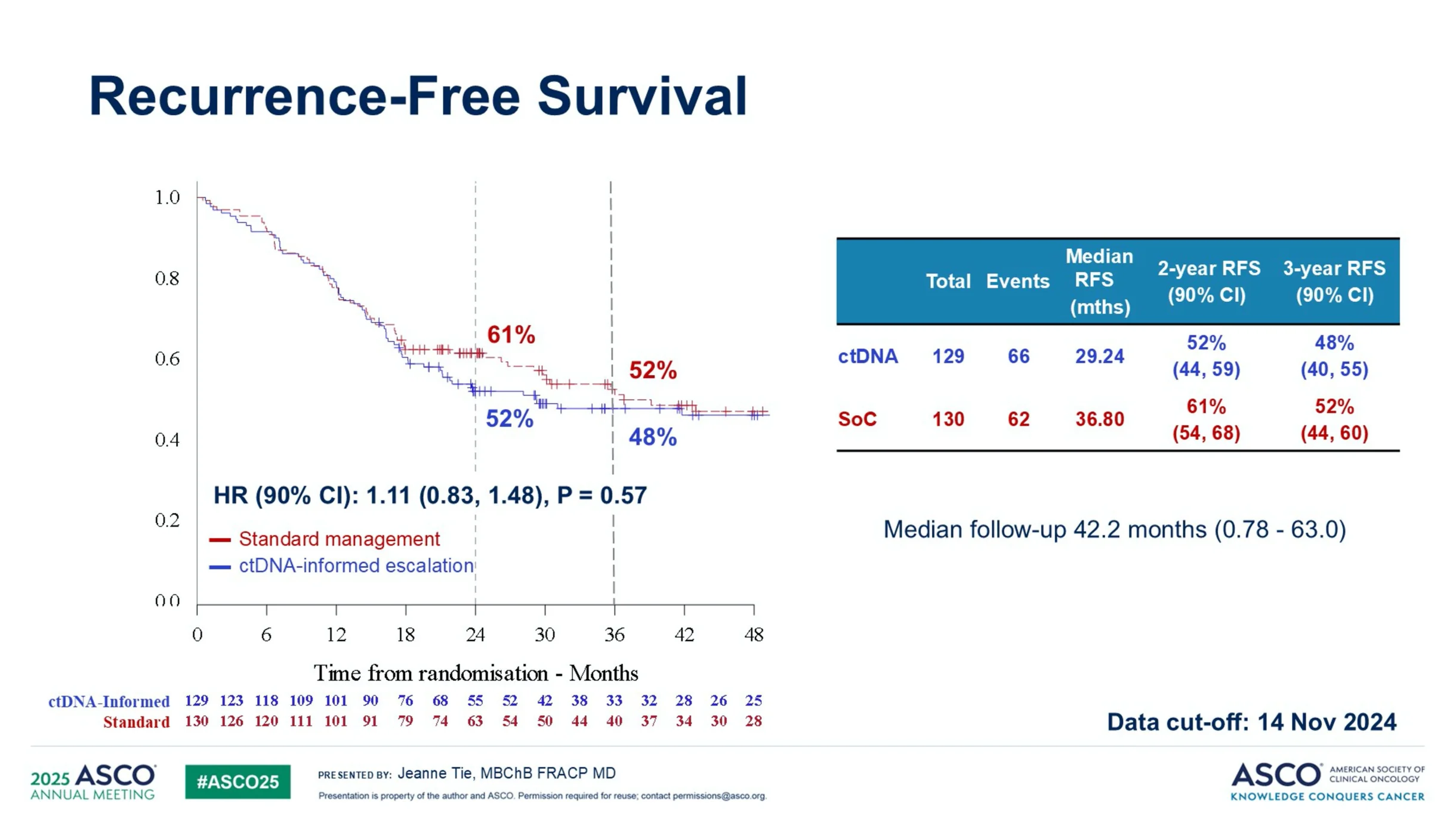
“Another highlight in 2025: DYNAMIC-III
No statistical significance in escalation for ctDNA+ or de-escalation for ctDNA-. Similarly, our ALTAIR failed to show DFS benefit for ctDNA+. Discussion must continue on utilizing ctDNA as a ‘strong prognostic & scientific tool’ optimally.”
Paolo Manca, MD – Medical Oncologist, Gastrointestinal Tumors
“Can we use an affordable method for ctDNA detection to monitor treatment in mCRC?
We’re excited to share our new manuscript in Clinical Cancer Research (AACR Journals), where we use METER – a low-pass whole genome bisulfite sequencing–based pipeline – to estimate dynamic ctDNA fraction in patients from the first-line VALENTINO trial.
This work was made possible by the work of Marta Paoli and Matteo Benelli, who developed METER, and – needless to say – to my mentor Filippo Pietrantonio, who envisioned this project and provided his constant guidance.”
Read full article here
Jennifer S. Buell – President and Chief Executive Officer, MiNK Therapeutics; Agenus; Tufts University School of Medicine
“A newly published analysis by ESMO – European Society for Medical Oncology by Bengala, Santini, Picone compares botensilimab + balstilimab with current standard-of-care therapies in refractory microsatellite-stable metastatic colorectal cancer.
Using reconstructed survival data from landmark SOC trials, the authors report a meaningful overall survival advantage and durability signal for BOT/BAL, despite heavy pretreatment, alongside a distinct toxicity profile compared with cytotoxic and TKI-based regimens. Importantly, outcomes appear strongly influenced by metastatic site, with markedly better survival in patients without active liver metastases—reinforcing emerging biology around immune exclusion and hepatic immune tolerance.
This work provides an independent, mechanism-consistent framework for patient selection, endpoint interpretation beyond early PFS, and future randomized validation. Notably, botensilimab + balstilimab has received compassionate access clearance in France, enabling near-term patient access while randomized studies continue.
Studies like this help move the field toward more biologically informed immunotherapy development in MSS colorectal cancer.”
Read full article here

Dimitrios Chatziisaak, MD – Doctor of Medicine (Dr. med.), MSc, MBA (candidate); Junior Member, Swiss College of Surgeons (SCS)
“Fresh out of the press!!
FLOT or CROSS? The debate continues.
Our new study is now published in the European Journal of Surgical Oncology (EJSO).
We compared the two main therapeutic strategies for esophageal cancer — #FLOT and #CROSS — in two pivotal studies — the #ESOPEC trial and real-world outcomes of the #CROSS regimen.
Key message:
FLOT demonstrates superior survival outcomes in controlled settings and should be considered in fit patients.
At the same time, CROSS remains an effective and well-tolerated real-world option.
Treatment choice matters — and should be driven by tumor location, patient fitness, and toxicity profiles.
Importantly, standardized pCR assessment is critical to meaningfully bridge clinical trials and real-world data.”
Read full article here

You can also read about 10 Must-Read Posts in GI Oncology from the Third week of December on OncoDaily.
