Fossils usually tell stories through shape and size. Bones show how animals moved, teeth hint at diet, and skulls suggest behavior. Now, scientists have found a new way to learn from fossils by studying chemistry locked inside ancient bones.
This…
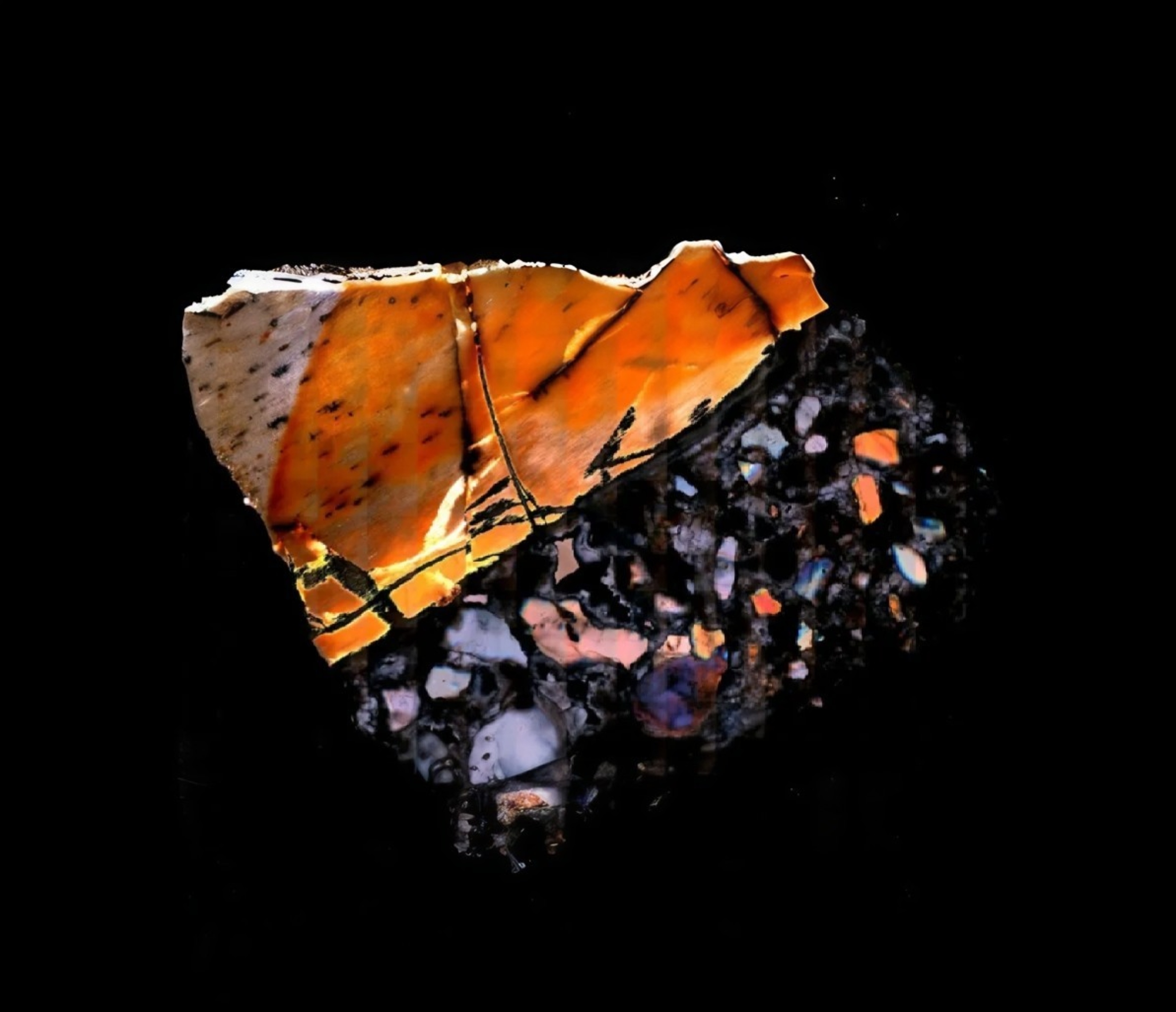
Fossils usually tell stories through shape and size. Bones show how animals moved, teeth hint at diet, and skulls suggest behavior. Now, scientists have found a new way to learn from fossils by studying chemistry locked inside ancient bones.
This…