A new electron diffraction method determines the room-temperature structures of solvated organic molecular microcrystals, according to a team of US-based researchers. The approach, which is presented in a preprint report and has not yet been peer reviewed, could make it easier for researchers to study molecules that need to remain solvated at all times, opening new opportunities in chemical and pharmaceutical research.
‘Many organic molecules bind water or depend on water for their structural stability, so placing such crystals in vacuum or even drying in air may alter their structure, giving erroneous results,’ explains Michael Elbaum at the Weizmann Institute of Science in Israel, who wasn’t involved in the study. He says that showing that electron diffraction can be measured on crystals embedded in liquid water is important. ‘I think that many will be surprised by this success,’ he says. ‘Room-temperature measurements in liquid water are often dismissed as hopeless, but the authors make a convincing case that their protocol solves the major issues.’
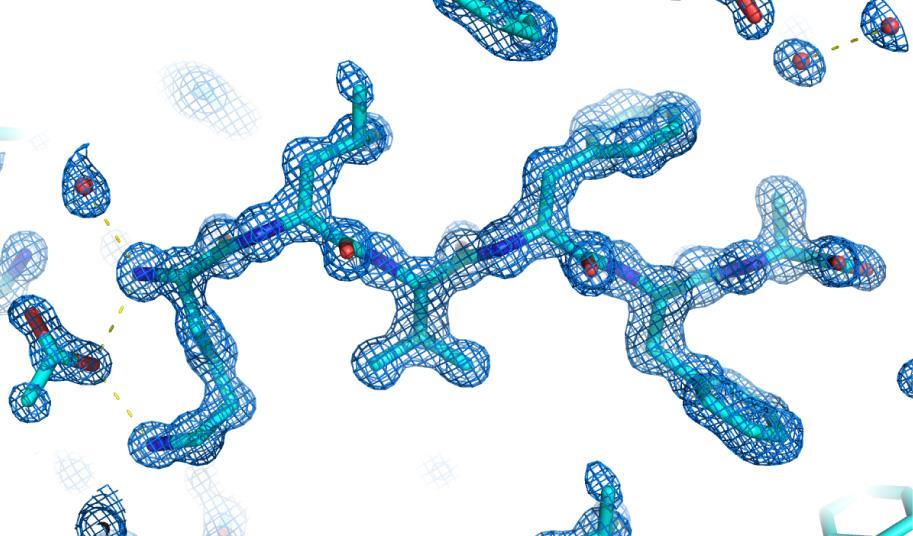
Microcrystal electron diffraction, or MicroED, is a powerful technique that uses high-energy electron beams (120–300eV) to illuminate tiny crystals. By analysing how these beams scattered, researchers can decode the samples’ molecular structures. ‘The interrogated crystals must resist the vacuum environment of the electron microscope and withstand the radiation from the electron beam,’ says Jose Rodriguez from the University of California, Los Angeles, in the US who led the project. He explains that freezing the samples – a strategy borrowed from cryo-EM – can help to mitigate these two effects, but only if the liquid surrounding the crystals can be frozen into glassy ice, which isn’t possible for all solvents.
‘We now show that this can also be achieved at room temperature on solvated crystals if we apply two innovations,’ says Rodriguez. The first of these strategies uses simple liquid cells to protect the crystal samples from vacuum damage. The second involves estimating a maximum tolerable exposure for each sample – to reduce damage by electron irradiation – and using new detectors and data acquisition techniques to extract as much signal as possible within that limit. ‘The detector timing to minimise beam damage was a bit of a challenge,’ Rodriguez admits. ‘It required automated software controls that have only recently become accessible.’
Rodriguez’s team tested several ways to keep crystals hydrated during their experiments and found that the best approach was to sandwich a thin liquid layer containing the crystalline samples between two thin sheets of carbon. Building the new liquid cells in this way is quite simple and only takes about 10 minutes, explains Rodriguez. ‘We use commercial transmission electron microscopy grids as the two layers of the sandwich, so that anyone can buy what they need to make them.’
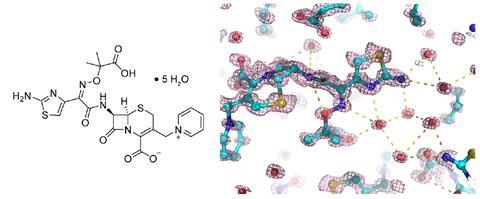
To obtain the cells, the researchers activated the two carbon grids using plasma and then placed a fraction of a microlitre of the crystal slurry between them, pressing them slightly together. The team tested the method on different organic molecules including solvated pharmaceuticals like the antibiotic ceftazidime and polypeptides.
‘This is not the most engineered or perfect approach, but it is very easy to implement and requires little specialised equipment for sample preparation. We hope it can be readily implemented by many labs around the world,’ notes Rodriguez. He says that existing methods that use sandwiches made from graphene layers are less convenient because they can produce signals that interfere with the crystallography measurements. ‘We opted to use amorphous carbon instead, which is easier to access, robust, and produces little diffraction background.’