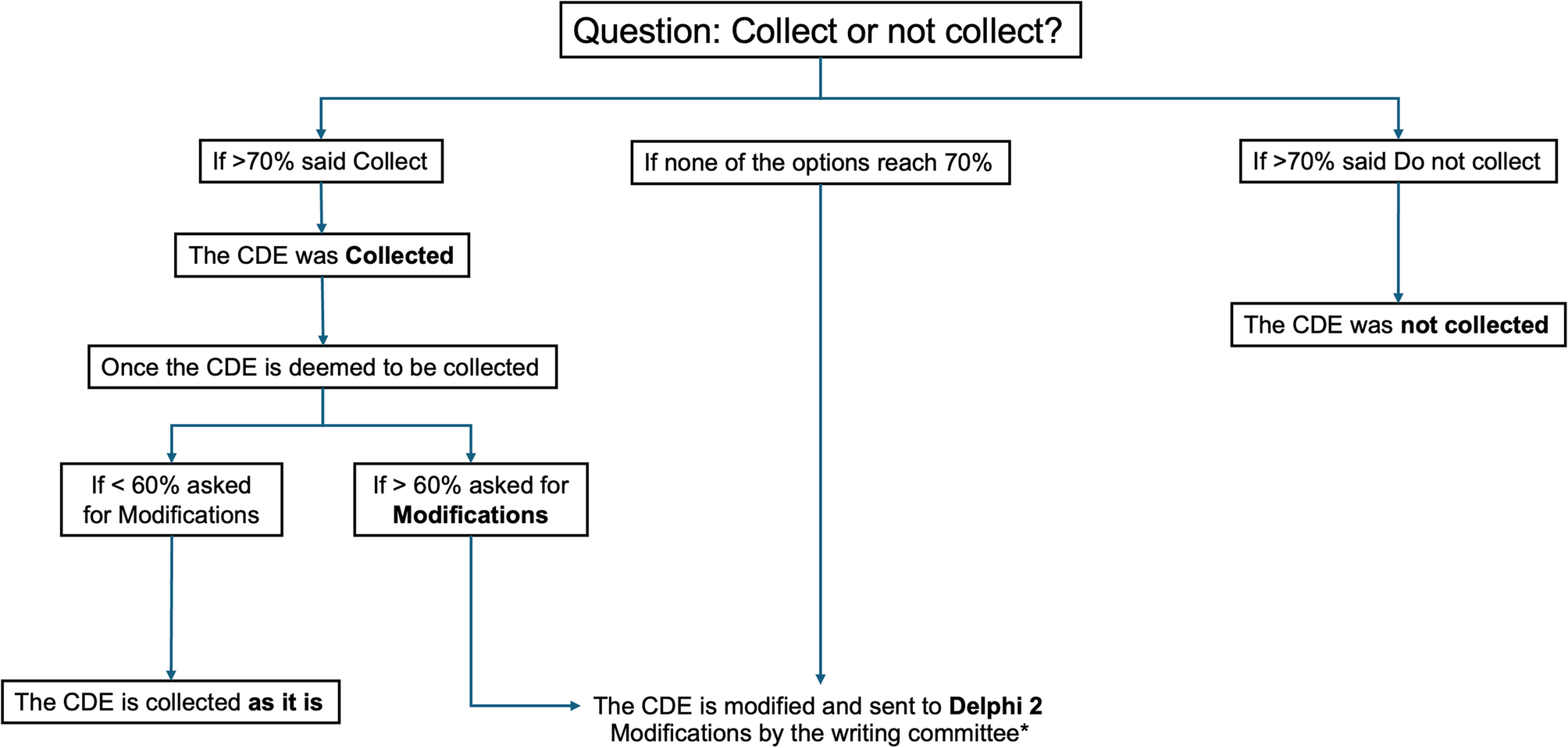
In this study, we employed a Delphi method to achieve expert consensus on a comprehensive set of CDEs for the study of OT. The high response rate among uveitis experts with extensive clinical experience strengthens the validity of our findings. Collectively, identifying and categorizing 139 CDEs—covering domains such as demographics, medical and ocular history, clinical presentation and findings, diagnostics, lesion characteristics, imaging findings, treatments, and outcomes—reflects OT’s complexity and multifaceted nature.
Our results demonstrate unanimous agreement that all 139 CDEs merit inclusion in a standardized CDE set, with nearly 80% rated very important (Table 5). These findings highlight a shared perception of their utility in guiding meaningful research and align with efforts in other medical fields to standardize data collection. Indeed, establishing standardized CDEs for OT provides an essential foundation for future research. By ensuring consistent data collection, these CDEs will facilitate comparability across studies, improving the ability to conduct meta-analyses and generate high-quality evidence for clinical guidelines [18]. Those CDEs rated as very important can serve as a core dataset for studies with limited resources, whereas the remaining CDEs can be used as optional variables depending on context, infrastructure, and study aims.
From an operational standpoint, incorporating standardized case report forms—drawing on existing frameworks such as the CDEs from the NIH/National Institute of Neurological Disorders and Stroke (NINDS)—could streamline data management and monitoring processes across different study arms [10, 19,20,21]. The CDEs provide a structured set of standardized definitions, formats, and protocols for collecting data, which ensures consistency and comparability across studies. For example, in traumatic brain injury research, CDEs have been instrumental in harmonizing data collection, enabling more robust data sharing and re-use, as highlighted by Hicks et al. [20] Similarly, CDEs have facilitated the systematic assessment of behavioral phenotypes in disorders of consciousness, as demonstrated by Yakhkind et al. [19] Furthermore, CDEs have been successfully adopted in assessing psychiatric comorbidities across brain disorders, underscoring their versatility and utility in diverse research contexts [21]. Following the NINDS example, the resulting set of CDEs for OT will be submitted to the NIH CDEs Repository to enhance their accessibility and findability [22].
Although vast research has been done on OT outcomes, leading to a better understanding of the disease [23,24,25], only a few studies have made their original data available. For instance, Sittivarakul et al. published their data on clinical characteristics, visual acuity outcomes, and factors associated with vision loss in patients with active OT at a Thai tertiary center [26]. While their dataset provides valuable insights into the disease’s clinical course, the absence of a standardized data dictionary limits its utility for broader integration into multicenter studies or patient-level meta-analyses. This exemplified the challenges associated with data reuse and integration in larger cohorts [27]. Without clear definitions and structured formats, reconciling variables across different datasets becomes challenging, hindering the ability to pool data or draw more robust conclusions. This underscores the importance of adopting standardized CDEs with well-defined dictionaries to ensure data consistency, interoperability, and reusability in ophthalmology research.
The interdisciplinary approach allowed for a comprehensive review of the questions to ensure clarity and ease of response and prevent ambiguities or leading statements. Additionally, response bias was mitigated by maintaining participant anonymity throughout the process, including during the analysis and the review of suggestions and changes. These measures collectively contribute to our study’s robustness and the reliability of its outcomes. These CDEs are designed to be compatible with electronic health records (EHRs) to facilitate implementation in real-world settings. They may be adapted into standardized case report forms or structured data entry templates. Once published and endorsed through the NIH Common Data Elements Repository, they will be better positioned for integration into clinical data systems and research platforms, supporting harmonization across institutions and improving data interoperability.
Despite these strengths, practical challenges remain in implementing such an extensive set. While this comprehensive pool of CDEs underscores the depth of information relevant to OT, not all variables may be feasible to collect in routine or resource-limited settings. Researchers may, therefore, need to tailor subsets of these CDEs to specific investigative goals or study designs [28]. Furthermore, the success of this endeavor requires ongoing collaboration and training across research centers to ensure adherence and uniformity in data collection. To address this, PROTON plans to pilot these CDEs in a multicenter observational study to refine them based on practical challenges and user feedback. Such a study is intended to be prospective, evaluating the impact of different treatment schemes and systemic corticosteroids on OT outcomes, including the effect of timing on disease progression and recovery.
Another consideration is the evolution of diagnostic and therapeutic modalities for OT [29]. As new technologies and treatments emerge, the relevance and perceived importance of certain CDEs may shift. Consequently, the CDE set identified here should be regarded as an adaptable resource, periodically updated in response to advancements in the field and feedback from real-world implementation. Another limitation of this study is that the study participants’ geographic distribution presents limitations due to confirmation bias. The majority of participating centres were located in South America (43%) and South Asia (30%), with limited representation from North America (3%), Europe (17%), East Asia (3%), and Oceania (3%). However, this distribution aligns with the regions where OT cases are most prevalent and where the greatest clinical needs exist, prioritizing perspectives from areas most affected by OT. Future studies should aim to include broader geographic representation, including North America, Europe, East Asia, and Oceania, in subsequent pilot or multicenter validations.
Beyond the foundational role of CDEs in standardizing data collection, these elements serve as a critical framework for enabling future research initiatives. By leveraging CDEs, researchers can design projects that integrate with existing ophthalmic and infectious disease databases, enhancing data interoperability and facilitating large-scale, multicenter analyses. Furthermore, the incorporation of omics data—such as genetic and immunological profiles—could provide deeper insights into host susceptibility and disease severity. Structured imaging annotation protocols, built upon CDEs, could also support AI-driven analyses for lesion classification, stratification, disease monitoring, and prediction of recurrence and resolution. Finally, systematic inclusion of longitudinal follow-up data will allow researchers to track recurrence rates, treatment efficacy, and disease progression over time, unlocking new avenues for understanding and managing the disease.
Moreover, expanding CDEs to encompass patient-reported outcomes (PROs) and quality-of-life (QOL) metrics will offer a more holistic assessment of disease burden, especially in chronic or recurrent cases, but it’s likely that PROs and QOLs will not need to be specific to OT and should engage a different cohort of subject matter experts and people with lived experience. Cost-effectiveness and healthcare utilization metrics should also be considered to evaluate treatment accessibility and economic impact. Pediatric-specific adaptations for congenital and early-onset OT, along with neurodevelopmental assessments, would further strengthen the applicability of CDEs across diverse patient populations. Training programs and dedicated research networks should be established to support the implementation of these CDEs, ensuring consistency, usability, and continuous refinement based on real-world feedback. Collectively, these enhancements will position OT CDEs as a dynamic and adaptable resource for advancing research, clinical care, and global collaboration in OT. To ensure broader applicability, future efforts should include the translation and cultural adaptation of CDE definitions into relevant local languages, particularly in endemic regions where OT prevalence is highest.
In conclusion, our Delphi study provides a robust starting point for a standardized OT data collection approach. These recommendations are intended to improve data interoperability, enabling data pooling to get more robust conclusions about disease progression, treatment, prophylaxis, prevention efficacy, and patient outcomes. Adopting these consensus-based CDEs can enhance comparability across studies, strengthen the evidence base for treatment strategies, and ultimately improve patient outcomes. Future endeavors should focus on practical implementation strategies, validation in varied clinical contexts, language translations, and iterative revisions to ensure this framework remains relevant and feasible.