The search for effective hydrogen storage materials represents a crucial step towards realising a sustainable, carbon-neutral energy future, and recent research introduces a novel nanomaterial with exceptional promise in this field. Bill D. Aparicio Huacarpuma, from the University of Brasília, alongside José A. S. Laranjeira and Nicolas F. Martins from São Paulo State University, and colleagues, present sodium-decorated Ennea-, a two-dimensional carbon allotrope constructed from interconnected carbon rings. Their investigations demonstrate that this material not only possesses remarkable mechanical and dynamic stability, but also exhibits an unprecedented capacity for hydrogen storage, exceeding the U. S. Department of Energy’s 2025 target. By strategically decorating Ennea- with sodium atoms, the team achieved reversible hydrogen adsorption, paving the way for the development of next-generation hydrogen storage technologies capable of operating under near-ambient conditions.
Sodium Enhances Ennea-Graphene Hydrogen Storage Stability
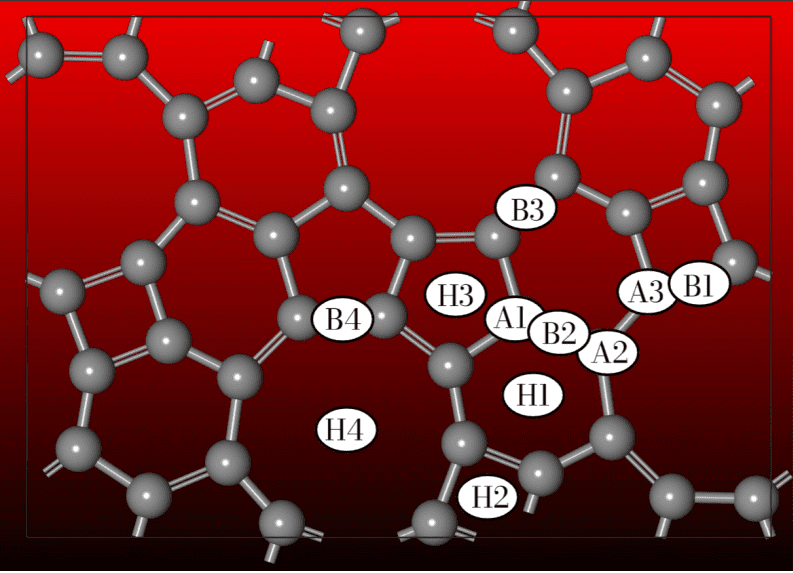
Scientists have pioneered a new two-dimensional carbon material, Ennea-Graphene, and demonstrated its exceptional potential for hydrogen storage. This innovative material, composed of interconnected four-, five-, six-, and predominantly nine-membered carbon rings, presents a unique porous framework distinct from existing 2D carbon materials like graphene and graphyne. Rigorous computational studies confirm Ennea-Graphene’s structural, mechanical, electronic, and thermal stability, paving the way for investigations into its hydrogen storage capabilities. Researchers employed density functional theory calculations to confirm the material’s stability, utilizing accurate modeling of electron behavior.
Phonon dispersion calculations validated the dynamic stability of Ennea-Graphene by demonstrating the absence of imaginary frequencies, confirming its inherent stability. Further analysis of elastic constants confirmed the material’s mechanical robustness, solidifying its potential for practical applications. The team discovered that decorating the Ennea-Graphene lattice with sodium atoms significantly enhances its hydrogen storage capacity. Sodium atoms preferentially bind to the material, forming a stable complex that acts as a catalyst and activation site for hydrogen adsorption. This allows the material to reversibly absorb a substantial amount of hydrogen, up to four hydrogen molecules per sodium atom, achieving a hydrogen storage capacity of 8.
These simulations confirmed the material remains stable even after saturating it with hydrogen, and that the adsorbed hydrogen can be released under near-ambient conditions. The adsorbed hydrogen maintains its molecular form throughout the process, indicating a physisorption mechanism that supports reversible storage. This unique material features a combination of four-, five-, six-, and nine-membered carbon rings, creating a porous and intricate framework unlike previously synthesized 2D materials. Computational studies demonstrate the material’s mechanical, thermal, and dynamic stability, establishing a strong foundation for exploring its hydrogen storage potential. Researchers employed density functional theory calculations to confirm the material’s stability, utilizing accurate modeling of electron behavior.
To further validate the material’s stability, the team calculated phonon dispersion relations, confirming the absence of imaginary frequencies and demonstrating Ennea-Graphene’s inherent stability. Elastic constants were also computed to evaluate mechanical robustness, further solidifying its potential for practical applications. Researchers then investigated the material’s hydrogen storage capacity by decorating the Ennea-Graphene lattice with sodium atoms. This research introduces a unique carbon architecture characterized by a combination of four-, five-, six-, and nine-membered carbon rings, creating a porous and intricate framework distinct from previously synthesized 2D materials. Calculations demonstrate the monolayer is both mechanically and dynamically stable at 300 K, exhibiting no indications of instability during rigorous testing. The material displays metallic-like electronic behavior and a high in-plane stiffness.
The team discovered that sodium atoms strongly bind to the Ennea-Graphene lattice, preserving its structural integrity even at room temperature. Crucially, the sodium-decorated structure exhibits exceptional hydrogen storage performance, reversibly adsorbing up to four hydrogen molecules per sodium atom. This translates to a hydrogen storage capacity of 8. 8 wt%, surpassing the U. S.
Ennea-Graphene Stores Hydrogen at Record Capacity
This research presents a novel two-dimensional carbon allotrope, Ennea-Graphene, and demonstrates its potential for high-capacity hydrogen storage. Calculations confirm the material’s mechanical, thermal, and dynamic stability, characterized by a unique combination of carbon rings and metallic-like electronic properties. The team discovered that sodium atoms strongly bind to the Ennea-Graphene lattice, preserving its structural integrity even at room temperature. Notably, the resulting sodium-decorated material exhibits exceptional hydrogen storage performance, reversibly adsorbing up to four hydrogen molecules per sodium atom, achieving a capacity of 8.
👉 More information
🗞 Sodium-Decorated Ennea-Graphene: A Novel 2D Carbon Allotrope for High-Capacity Hydrogen Storage
🧠 ArXiv: https://arxiv.org/abs/2509.19060