Researchers have captured the living mechanics of hearing for the first time by sustaining a piece of cochlear tissue outside the body.
Shortly before his death in August 2025, A. James Hudspeth and his colleagues at The Rockefeller University’s Laboratory of Sensory Neuroscience accomplished a milestone that had never been reached before. They succeeded in keeping a small section of the cochlea alive and working outside the body, making it possible to study the organ’s function directly for the first time. Using a specially designed device, the team was able to track the cochlea’s extraordinary abilities in real time, including its fine-tuned sensitivity, precise frequency detection, and capacity to process a wide range of sound levels.
“We can now observe the first steps of the hearing process in a controlled way that was previously impossible,” says co-first author Francesco Gianoli, a postdoctoral fellow in the Hudspeth lab.
The achievement, detailed in two recent publications (in PNAS and Hearing Research, respectively), represents the culmination of Hudspeth’s fifty years of pioneering research into the cellular and neural basis of hearing. His work has continually pointed toward new possibilities for preventing and treating hearing loss.
Beyond its immediate applications, the advance also delivers long-sought experimental confirmation of a fundamental biophysical principle that underlies hearing across diverse species, a concept Hudspeth had pursued for more than twenty-five years.
“This study is a masterpiece,” says biophysicist Marcelo Magnasco, head of the Laboratory of Integrative Neuroscience at Rockefeller, who collaborated with Hudspeth on some of his seminal findings. “In the field of biophysics, it’s one of the most impressive experiments of the last five years.”
The mechanics of hearing
Though the cochlea is a marvel of evolutionary engineering, some of its fundamental mechanisms have long remained hidden. The organ’s fragility and inaccessibility—embedded as it is in the densest bone in the body—have made it difficult to study in action.
These challenges have long frustrated hearing researchers, because most hearing loss results from damage to sensory receptors called hair cells that line the cochlea. The organ has some 16,000 of these hair cells, so-called because each one is topped by a few hundred fine “feelers,” or stereocilia, that early microscopists likened to hair. Each bundle is a tuned machine that amplifies and converts sound vibrations into electrical responses that the brain can then interpret.
It’s well documented that in insects and non-vertebrate animals—such as the bullfrogs studied in Hudspeth’s lab—a biophysical phenomenon known as a Hopf bifurcation is key to the hearing process. The Hopf bifurcation describes a kind of mechanical instability, a tipping point between complete stillness and oscillations. At this knife-edge, even the faintest sound tips the system into movement, allowing it to amplify weak signals far beyond what would otherwise register.
In the case of bullfrog cochlea, the instability is in the bundles of the sensory hair cells, which are always primed to detect incoming sound waves. When those waves hit, the hair cells move, amplifying the sound in what’s called the active process.
In collaboration with Magnasco, Hudspeth documented the existence of the Hopf bifurcation in the bullfrog cochlea in 1998. Whether it exists in the mammalian cochlea has been a subject of debate in the field ever since.
To answer that question, Hudspeth’s team decided they needed to observe the active process in a mammalian cochlea in real time and at a greater level of detail than ever before.
A sliver of a spiral
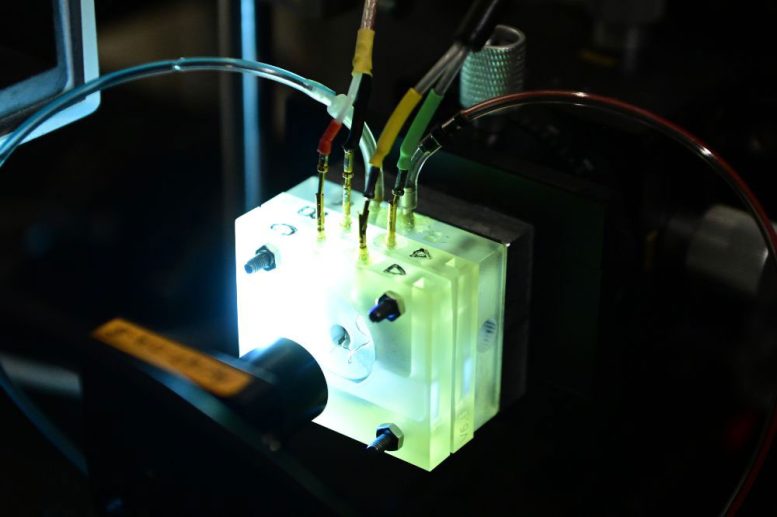
To do so, the researchers turned to the cochlea of gerbils, whose hearing falls in a similar range as humans. They excised slivers no larger than .5 mm from the sensory organ, in the region of the cochlea that picks up the middle range of frequencies. They timed their excision to a developmental moment in which the gerbil’s hearing is mature but the cochlea hasn’t fully fused to the highly dense temporal bone.
They placed a sliver of tissue within a chamber designed to reproduce the living environment of the sensory tissue, including continuously bathing it in nutrient-rich liquids called endolymph and perilymph and maintaining its native temperature and voltage. Key to the development of this custom device were Brian Fabella, a research specialist in the Hudspeth lab, and instrumentation engineer Nicholas Belenko, from Rockefeller’s Gruss Lipper Precision Instrumentation Technologies Resource Center.
They then began to play sounds via a tiny speaker and observed the response.
Discovering a biophysical principle
Among the processes they witnessed were how the opening and closing of ion channels in the hair bundles add energy to the sound-driven vibrations, amplifying them, and how outer hair cells elongate and contract in response to voltage changes through a process called electromotility.
“We could see in fine detail what every piece of the tissue is doing at the subcellular level,” Gianoli says.
“This experiment required an extraordinarily high level of precision and delicacy,” notes Magnasco. “There’s both mechanical fragility and electrochemical vulnerability at stake.”
Importantly, they observed that the key to the active process was indeed a Hopf bifurcation—the tipping point that turned mechanical instability into sound amplification. “This shows that the mechanics of hearing in mammals is remarkably similar to what has been seen across the biosphere,” says co-first author Rodrigo Alonso, a research associate in the lab.
A device that could lead to future treatments
The scientists anticipate that experimentation using the ex vivo cochlea will enhance their understanding of hearing and potentially lead to more effective therapies.
“For example, we will now be able to pharmacologically perturb the system in a very targeted way that has never been possible before, such as by focusing on specific cells or cell interactions,” says Alonso.
There’s a great need in the field for new potential therapies. “So far, no drug has been approved to restore hearing in sensorineural loss, and one reason for that is that we still have an incomplete mechanistic understanding of the active process of hearing,” Gianoli says. “But now we have a tool that we can use to understand how the system works, and how and when it breaks—and hopefully think of ways to intervene before it’s too late.”
Hudspeth found the results deeply gratifying, Magnasco adds. “Jim had been working on this for more than 20 years, and it’s a crowning achievement for a remarkable career.”
References:
“Amplification through local critical behavior in the mammalian cochlea” by Rodrigo G. Alonso, Francesco Gianoli, Brian Fabella and A. J. Hudspeth, 14 July 2025, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2503389122
“Toward an ex vivo preparation for studies of the cochlear active process in mammals” by Francesco Gianoli, Rodrigo Alonso, Brian Fabella and A.J. Hudspeth, 24 April 2025, Hearing Research.
DOI: 10.1016/j.heares.2025.109288
Never miss a breakthrough: Join the SciTechDaily newsletter.