Stafford, Texas – September 10, 2025 — Greenwich LifeSciences, Inc. (Nasdaq: GLSI), a clinical-stage biopharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for its lead immunotherapy candidate GLSI-100 in HLA-A*02–positive, HER2-positive breast cancer patients who have completed standard HER2-targeted therapy.
The designation recognizes GLSI-100’s potential to improve invasive breast cancer–free survival and addresses a significant unmet need in preventing metastatic recurrence.
You Can Read About Breast Cancer: Symptoms Causes, Stages, Diagnosis and Treatment
How It Works
GLSI-100 is designed to “train” the immune system to recognize and attack HER2-expressing cancer cells that may remain after surgery and standard HER2-targeted therapy (such as trastuzumab). By stimulating a long-lasting cytotoxic T-cell response, it aims to reduce the risk of cancer relapse and progression to metastatic disease.
Why This Matters
FDA’s Fast Track program is designed to accelerate development and review of therapies for serious conditions with unmet needs. For GLSI-100, the designation allows:
- More frequent FDA communication to optimize clinical and regulatory pathways.
- Rolling review of the Biologic License Application (BLA), enabling sections of the application to be submitted and reviewed as they are completed.
- Potential eligibility for Accelerated Approval and Priority Review.
As Dr. Jaye Thompson, VP of Clinical and Regulatory Affairs at Greenwich, noted:
“The FDA sees the potential of GLSI-100 to change important clinical outcomes in this population of breast cancer patients.”
Phase IIb Results: A Strong Foundation
The FDA decision builds on promising data from a Phase IIb randomized, placebo-controlled trial:
- 80% or greater reduction in metastatic breast cancer recurrence after 5 years.
- Peak immune response achieved at 6 months, sustained with booster doses.
- No serious adverse events attributable to GLSI-100.
- Favorable safety and tolerability profile across 146 patients in Phase I/II trials.
These results suggest GLSI-100, based on the GP2 peptide combined with GM-CSF, could offer durable protection against recurrence in high-risk HER2-positive patients.
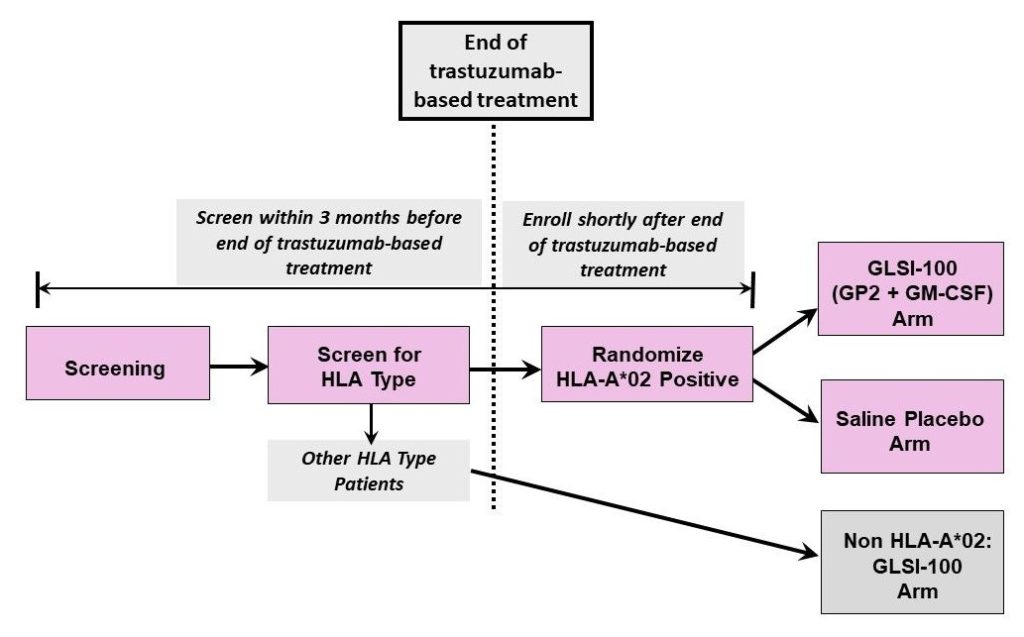
The Phase III FLAMINGO-01 Trial
The ongoing FLAMINGO-01 Phase III trial (NCT05232916) aims to confirm GLSI-100’s efficacy and safety in a larger global cohort.
- ~500 HLA-A*02 patients randomized to GLSI-100 or placebo.
- Additional 250 patients of other HLA types receiving GLSI-100 in an open-label arm.
- Led by Baylor College of Medicine, with plans for 150 clinical sites worldwide.
- Powered to detect a hazard ratio of 0.3 in invasive breast cancer–free survival.
An interim analysis will assess superiority and futility after 14 events, ensuring early insights into clinical benefit.
A New Frontier in Breast Cancer Immunotherapy
HER2-positive breast cancer remains one of the most aggressive subtypes, with high risk of recurrence despite targeted therapy. GLSI-100 represents a novel immunotherapy approach designed to prevent relapse by training the immune system to recognize and eliminate residual HER2-expressing tumor cells.
CEO Snehal Patel summarized the potential impact:
“By showing the potential of GLSI-100 to prevent metastatic breast cancer recurrence, we were able to estimate the potential lives that could be saved.”
The company plans to engage further with FDA and European regulators to expand access to broader patient populations.
About Greenwich LifeSciences
Greenwich LifeSciences is advancing GP2-based immunotherapy for preventing breast cancer recurrence. Its lead program, GLSI-100, combines the GP2 peptide—derived from the HER2 protein—with GM-CSF to generate durable immune responses. The company is committed to reducing recurrence risk in patients who have already undergone surgery and HER2-targeted therapy.
Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO
