Echocardiography
Echocardiography, especially transthoracic echocardiography (TTE), is considered a primary screening and diagnostic tool for patients with congenital heart disease. In fact, in the majority of cases with isolated TAPVC, TTE alone provides sufficient information for straightforward surgical intervention [3, 4].
Diagnosing TAPVC through echocardiography relies on identifying key features such as the absence of pulmonary venous connection to the left atrium, the presence of CPVT behind the left atrium, and its various connections to the right atrium. Each type exhibits distinct characteristics, which can be discerned in echocardiography by observing the course and location of TAPVC and any obstruction in pulmonary venous inflow [9]. Supracardiac TAPVC can be identified using the suprasternal long-axis rotation cut, while infracardiac TAPVC is best visualized with the subcostal short-axis rotation cut. Coronary sinus TAPVC can be distinguished from cor triatriatum using the parasternal long-axis and apical four-chamber views (Fig. 1), along with their tilt-axis views. A misalignment between the underdeveloped septum secundum and the leftward-displaced septum primum, known as septum primum malposition defect, can lead to TAPVC draining directly into the right atrium. Moreover, understanding the precise nature of this anomaly preoperatively is crucial for effective surgical management [25].
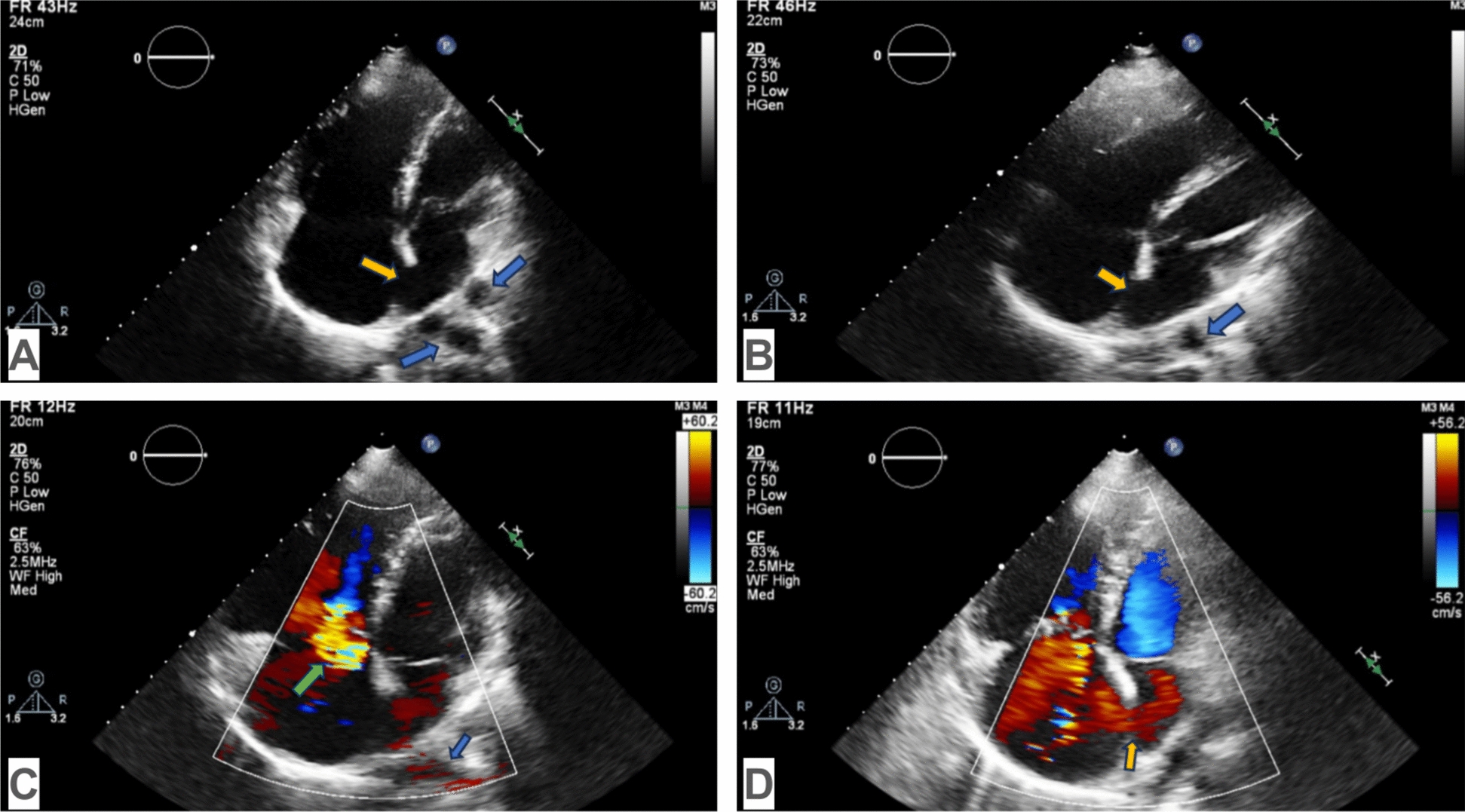
Echocardiography in TAPVC can visualize a hypoplastic left atrium since the pulmonary veins, the common collector of the pulmonary veins, do not drain into the left atrium. Tricuspid valve regurgitation, right heart dilatation, and atrial septal defect may also be detected: A apical four-chamber view (M and B modes): dilation of the right heart and hypoplasia of the left heart, atrial septal defect (yellow arrow), pulmonary veins (blue arrows); B apical four-chamber view (M and B modes): dilatation of the right heart and hypoplasia of the left heart, atrial septal defect (yellow arrow), common pulmonary venous trunk (blue arrow); C color Doppler apical four-chamber view (M and B modes): tricuspid valve regurgitation (green arrow), common pulmonary venous trunk (blue arrow); D color Doppler apical four-chamber view (M and B modes): right to left shunt blood flow in an atrial septal defect (yellow arrow)
2D echocardiography with Doppler interrogation is a highly reliable method for diagnosing TAPVC, as it can identify pulmonary vein flow and confirm anomalous connection to the right atrium [9, 25]. Nonphasic venous flow observed on pulsed Doppler echocardiography indicates the presence of an obstruction [16]. Additionally, false-positive interpretations of mixed variety caused by artifacts can be avoided by verifying with Doppler images or conducting repeated studies [26].
However, TEE has some limitations, like a limited field of view (it is not always possible to trace each pulmonary vein and follow its drainage site) [27], poor echocardiographic acoustic windows, limited spatial resolution, and operator dependence [26]. Therefore, CTA or MRI can help in the precise delineation of the pulmonary venous connections and evaluation of cardiac and extracardiac structures, as in cases of heterotaxy syndromes [3], suspicion about the presence of mixed TAPVC [28]. Thus, cross-sectional imaging is typically reserved for cases where the diagnosis is uncertain or the PVs are incompletely defined by echocardiography [3].
Computed tomography angiography
Computed tomography angiography offers an alternative diagnostic technique with excellent spatial and temporal resolution for the detailed preoperative anatomical evaluation of TAPVC (Fig. 2). Additionally, it assists in identifying airway abnormalities [17].
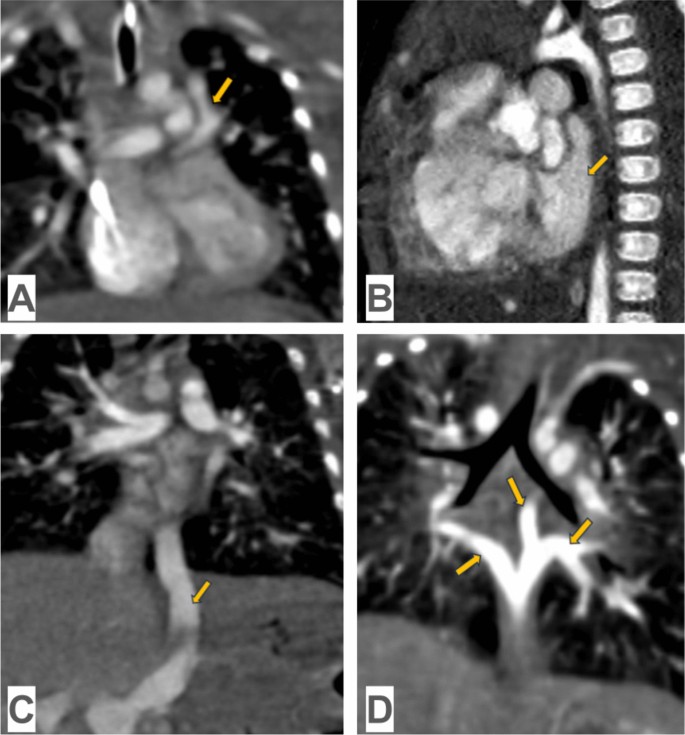
Computed tomography angiography in TAPVC: A supracardiac TAPVC: common pulmonary venous trunk, which connects to the innominate vein with obstruction in the frontal view (yellow arrow); B cardiac TAPVC: common pulmonary venous trunk, which drains into the coronary sinus in the sagittal view (yellow arrow); C infracardiac TAPVC: common pulmonary venous trunk, which connects to the hepatic vein in the frontal view (yellow arrow); D mixed TAPVC: the right inferior pulmonary vein and the left pulmonary veins, which are connected to form a common trunk in the frontal view (yellow arrow)
CTA may not offer additional diagnostic information for isolated TAPVC but is considered superior to TTE and catheter angiography for evaluating complex TAPVC [12]. Also, CTA has a leading role in preoperative planning by offering detailed anatomical information for patients with a biventricular heart and enabling visualization of anatomical variations, which leads to a significant survival benefit [29]. Furthermore, CTA can depict the path of anomalous PVs from the periphery, through the hilum and mediastinum, to their connection sites. It is beneficial for visualizing abnormal vascular connections outside the usual echocardiographic windows [12]. In critically ill neonates with obstructive TAPVC, computed tomography (CT) can be especially useful for treatment planning [3].
To achieve isotropic reformatted and 3D volume-rendered images in CTA (Fig. 3), it is recommended to use the thinnest possible detector collimation (or detector configuration). Nonionic low-osmolality (or iso-osmolality) iodinated contrast material is administered intravenously at a dose of 1.5–2.0 mL/kg (up to a maximum of 120–150 mL), typically using a power injector with injection rates ranging from 1 to 4 mL/s, adjusted according to patient weight and the quality of intravenous access [30]. For example, in a 10-kg child receiving 1.5 mL/kg of contrast agent, the injection rate would be set at 1.0 mL/s [31].
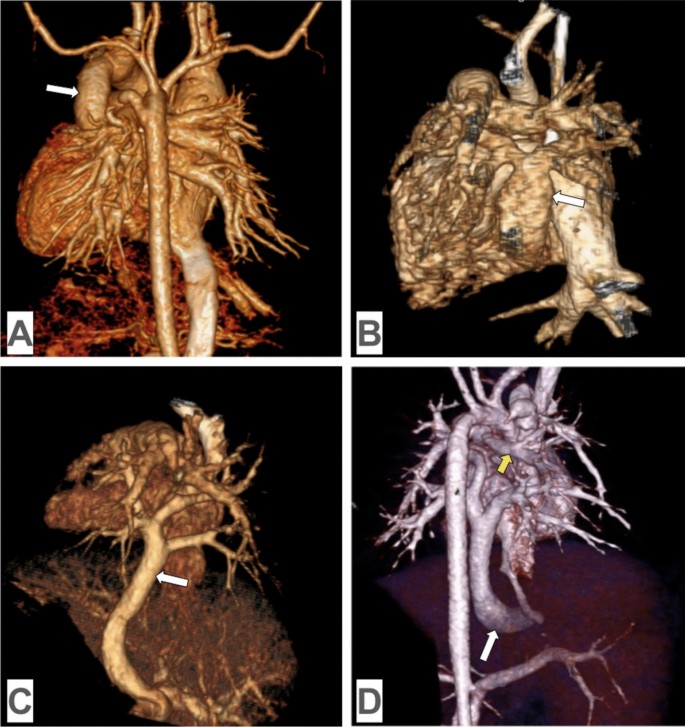
3D volume-rendered images from computed tomography angiography in TAPVC: A supracardiac TAPVC: all four pulmonary veins merging to form a common pulmonary venous trunk (white arrow), which drains into the superior vena cava through the innominate vein in the posterior view; B cardiac TAPVC: all four pulmonary veins merging to form a common pulmonary venous trunk (white arrow), which drains into the coronary sinus in the posterior view; C infracardiac TAPVC: all four pulmonary veins merging to form a common pulmonary venous trunk (white arrow), which passes through the diaphragm and drains into the the portal vein in the posterior view; D mixed TAPVC: the right inferior pulmonary vein and the left pulmonary veins merging to form a common collector (white arrow), which drains into the inferior vena cava, while the right superior pulmonary vein (yellow arrow) drains into the innominate vein in the posterior view
CT protocols for congenital heart disease are tailored by age to balance diagnostic quality with radiation safety. In children under five, non-ECG-gated or high-pitch spiral acquisitions are typically sufficient for evaluating extracardiac structures [32], with ECG-gated scans reserved for detailed intracardiac or coronary assessment [33]. Sedation is often required in this age group. For children over five, improved cooperation allows routine use of prospective ECG-gated scans [31], with retrospective gating used when functional or multiphasic imaging is needed. In adults, ECG-gated CT is standard, with prospective gating preferred to reduce radiation and retrospective gating employed for functional evaluation or irregular rhythms [34]. Across all age groups, dose optimization strategies such as low kVp, automated exposure control, and iterative reconstruction are essential [35].
The superior spatial resolution of CT and its ability to assess extracardiac structures such as the great vessels make it the preferred technique for producing 3D models of congenital heart disease. These models assist clinicians in better visualizing anatomical anomalies and have the potential to influence surgical outcomes, increasing their desirability and possibly leading to their routine use in clinical practice [36].
CTA addresses the limitations of TTE, such as suboptimal acoustic windows and inadequate visualization of extracardiac vascular structures. It also surpasses catheter angiography, which often produces overlapping views of adjacent vessels, complicates the simultaneous depiction of the systemic and pulmonary vascular systems, leads to undesirable catheter-related complications, and involves high ionizing radiation doses [4]. Additionally, CTA is more cost-effective and efficient than MRI, which needs extended image acquisition and patient sedation times [17]. These superiorities of CTA over other imaging modalities make it indispensable in diagnosing TAPVC.
Due to the significant concern of radiation exposure from CTA, particularly in children, and the associated risks of radiation-induced DNA damage and cancer, it is crucial to minimize radiation dosage without compromising image quality. Clinicians need to keep radiation “as low as reasonably achievable” consistently [4]. To address this limitation, current-generation multi-slice CT scanners allow for substantial radiation dose reduction by using high-pitch acquisition modes, with values reaching up to 3.4 on dual-source systems [37], or by employing target mode acquisitions. These advanced techniques enable imaging within a single or a few heartbeats, capturing data during a specific portion of the cardiac cycle, as seen in prospective ECG-gated scanning [34].
However, despite its main disadvantage of radiation, CTA is becoming progressively crucial in diagnosing TAPVC [17].
Magnetic resonance imaging
Cardiac MRI is a valuable technique in diagnosing and managing TAPVC, enabling anatomical and physiological assessment, evaluation of ventricular function and volumes, quantification of shunt ratios (Qp: Qs), and three-dimensional multiphase angiographic imaging without exposing patients to ionizing radiation [38,39,40]. It complements other diagnostic imaging tools, such as echocardiography and CTA, contributing to a thorough assessment of patients with suspected or confirmed TAPVC [41]. Furthermore, MRI can provide extensive cardiovascular anatomy and physiology detailing as valuable as CTA [42], aiding in the planning for transcatheter and surgical therapies [43]. In addition, in older patients with less definitive indications for surgery, the functional insights offered by cardiac MRI can be particularly useful for evaluating the hemodynamic significance of pulmonary venous anomalies [44].
Conventional MRI techniques, such as spin and gradient-echo sequences, have demonstrated precise delineation of pulmonary and systemic vein anomalies [45,46,47] (Fig. 4). However, traditional white-blood imaging sequences may not consistently provide sufficient spatial resolution, especially for complex anatomy in peripheral PVs. Contrast-enhanced magnetic resonance angiography (MRA) and time-resolved MRA significantly improve visualization capabilities in such cases. Nonetheless, these methods also require extended scanning times. Phase-contrast MRI can also be utilized to analyze flow patterns within the PVs [38].
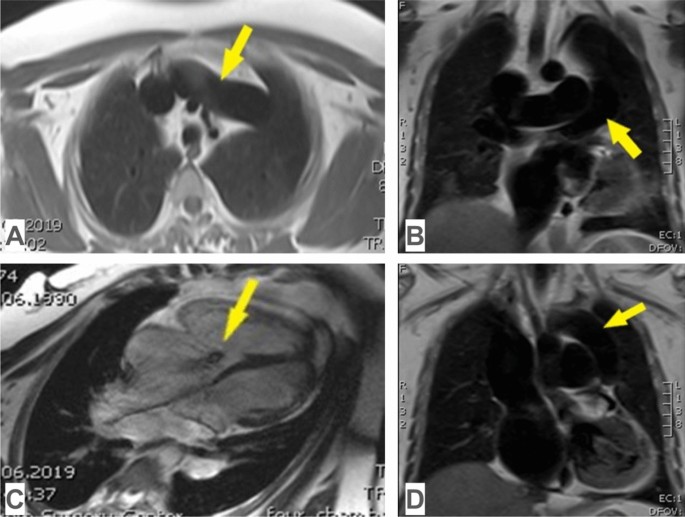
Magnetic resonance imaging in supracardiac TAPVC: A common pulmonary venous trunk, which connects to the innominate vein in the axial view (yellow arrow); B common pulmonary venous trunk in frontal view (yellow arrow); C tricuspid valve regurgitation (yellow arrow), hypoplasia of the left heart, dilatation of the right heart in a four-chamber view; D common pulmonary venous trunk, which connects to the innominate vein in the frontal view (yellow arrow)
Gradient-recalled echo, particularly 2D-balanced steady-state free precession (SSFP) pulse sequences, generates bright blood cine images assisting in assessing cardiac chambers and valvular function [30, 48]. These sequences are also effective for evaluating the anatomy of the central PVs and the left atrium. Respiratory-triggered electrocardiogram-gated free-breathing 3D-balanced SSFP pulse sequences enable the acquisition of nearly isotropic volumetric data, which can be reformatted to acquire a 3D whole-heart MRI and examined in various planes [49]. This technique allows precise segmental anatomical assessment and supports a wide range of applications, including advanced sequence planning, volumetric analysis of complex cardiac geometries, 3D printing for surgical guidance, and computational modeling [50].
The MRI protocol for TAPVC primarily focuses on assessing the pulmonary and systemic vasculatures. Given that patients with TAPVC are often neonates who may be clinically unstable, conducting a brief and effective MRI study is crucial. The protocol typically includes axial cine gradient-echo imaging with a section thickness of 3 to 4 mm and overlapping slices to minimize volume averaging, along with thin-section black-blood imaging in axial and/or coronal planes of the chest. High-resolution gadolinium-enhanced 3D MRA is employed to delineate the anomalous venous drainage [3], with contrast administered either manually or via power injection at rates below 1–2 mL/s, often using a double dose (0.20 mmol/kg) to enhance vascular definition [51, 52]. In older children and adults, or postoperative cases, additional sequences such as breath-hold cine SSFP, phase-contrast imaging for flow quantification, and late gadolinium enhancement may be incorporated to assess ventricular function, pulmonary venous obstruction, and myocardial viability [53]. In advanced settings, the volumetric coverage provided by 4D flow MRI may be incorporated to assess complex hemodynamics [54, 55].
MRI offers several advantages, such as the absence of ionizing radiation, multiplanar imaging capability, natural soft tissue contrast, quantitative flow analysis, and the ability to capture multiple vascular phases with a single injection of gadolinium contrast material [42]. Importantly, MRI examinations can be tailored to address specific clinical questions, allowing for a more focused protocol that can significantly reduce the overall scan time without compromising diagnostic value [56, 57]. Despite these benefits, the MRI technique is limited by the extended durations required for comprehensive anatomical coverage [43] and its tendency to produce metal-related artifacts [30].
Cardiac catheterization
Over the past 20 years, echocardiography has emerged as the leading noninvasive method for diagnosing venous anomalies, while cardiac catheterization remains the benchmark diagnostic procedure [58, 59], primarily in patients with complex TAPVC for additional anatomic and functional characterization [12] (Fig. 5).
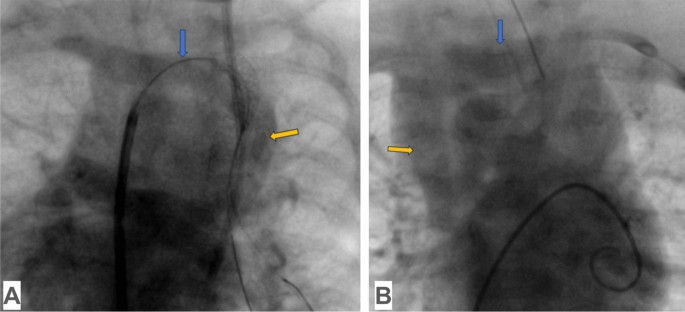
Cardiac catheterization in supracardiac TAPVC: A common pulmonary venous trunk, which connects to the innominate vein without obstruction (yellow arrow), innominate vein (blue arrow); B innominate vein (blue arrow), superior vena cava (yellow arrow)
Right heart catheterization is an invasive procedure used to directly measure pressures within the right side of the heart, oxygen saturations within the cardiac chambers, and calculation of cardiac output [60]. Specifically, right heart catheterization with delayed-phase pulmonary angiography allows for a thorough assessment of PV connection and potential obstructions, but it may not consistently visualize small accessory and anomalous vessels [42].
While conventional catheter angiography has long been regarded as the gold standard, it is an operator-dependent and costly procedure [61]. Additionally, it poses risks such as cardiac arrest and even death in cases of obstructive TAPVC and severe cyanosis [4] due to its invasive nature, exposure to ionizing radiation, and use of iodinated contrast agents [43].
Therefore, cardiac catheterization is generally excessive when diagnosing TAPVC because of its numerous disadvantages. Instead, noninvasive diagnostic modalities like CTA and MRI are routinely employed and are often considered superior to cardiac catheterization [42].