Quantitative results
Overview of the inclusion documents
Our comprehensive search and screening in WHO databases captured 28 relevant documents, including 11 WHA resolutions,17 EB decisions and annexes covering global health issues, particularly tuberculosis, HIV and antimicrobial resistance. The specific issues related to tuberculosis, HIV and antimicrobial resistance among the 28 documents are summarized in Table 3. TB has 3 resolutions and 5 EB decisions; HIV has 1 resolution and 3 EB decisions, which is the least issues; AMR has 4 resolutions and 7 EB decisions, which owns the most attention compared with TB and HIV.
The frequency of the keywords
The keywords frequencies of tuberculosis, HIV, antimicrobial resistance and one health in different resolutions and decisions are summarized in Table 4. The keyword “Tuberculosis” appears with varying frequencies across documents. For instance, it is mentioned 167 times in document EB150/9, which is the highest count for TB in the provided data. However, in documents EB146/11 and EB148/11, TB is not mentioned at all. The keyword “HIV” shows a high count in document A69/31 with 590 mentions, indicating a strong focus on this topic in that particular resolution or decision. Document EB138/29 also has a significant number of mentions (111). Conversely, there are documents like EB148/37 and EB150/8 with relatively fewer mentions. The keyword “antimicrobial resistance” is most frequently mentioned in document EB136/20 with 111 occurrences, suggesting a concentrated discussion on the topic in that document. Document A68/20 also has a high count of 115 mentions. However, in documents like EB148/11 and EB154/13, the keyword is mentioned only once. The co-occurrence is relatively low, with the highest being in document A68/20, EB136/20,and EB140/11, where it happens twice, and several documents show no instances of all three keywords appearing together. The data suggests that the focus on each keyword varies significantly across different resolutions and decisions, which reflects the priorities and topics of discussion during the respective sessions. The high frequency of mentions for a particular keyword in some documents could indicate a stronger emphasis on that health issue during those sessions. The lack of co-occurrence of all three keywords and one health in many documents might suggest that while these issues are discussed separately, there may be opportunities for more integrated discussions that consider the interrelated nature of these global health challenges.
Broader policy trends (2015–2024)
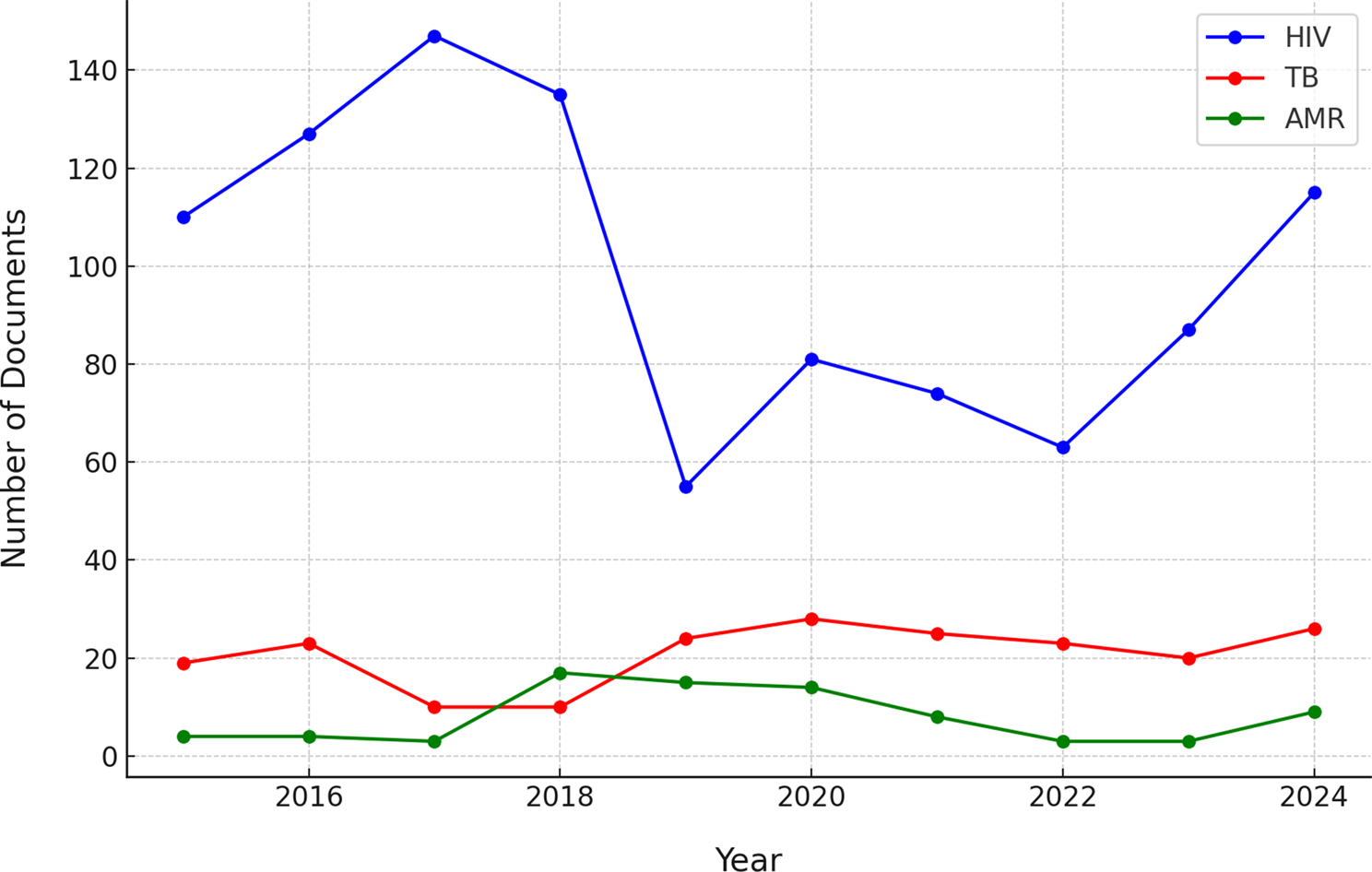
In addition to WHO resolutions and decisions, we examined the broader landscape of policy documents pertaining to tuberculosis (TB), HIV, and antimicrobial resistance (AMR) issued globally between 2015 and 2024. Analysis of the Cortellis regulatory intelligence database revealed divergent trends in the volume of policy activity for each issue (Fig. 1). HIV-related policy and regulatory documents were issued in substantially greater numbers annually compared to those addressing TB or AMR. HIV documents peaked in 2018 with 150 issued that year, driven by numerous approvals of antiretroviral drugs, guidelines, and program policies; subsequently, issuance declined slightly and stabilized at 90–110 documents annually through 2024. TB-related policy documents were considerably fewer per year (typically 7 to 26), but exhibited surges in 2019 and again during 2023–2024, potentially corresponding to renewed global TB commitments and the introduction of new diagnostic or treatment guidelines. AMR-related policy documents were minimal prior to 2017, then increased, with a notable peak in 2022 when approximately 30 AMR policy documents were issued globally. The cumulative volume over the decade underscores a stark imbalance: 1,103 HIV-related policy documents versus 144 for TB and 120 for AMR. This pattern illustrates that HIV has been the subject of far greater policy and regulatory activity, reflecting its longstanding prioritization on the global health agenda.The synchronized 2019 peaks for HIV and TB (and sustained high HIV output in 2024–25) may reflect broader policy windows (e.g., waves of approvals/generics/indications or ecosystem changes). These windows can accelerate cross-national alignment through reference or reliance pathways.
Annual number of policy/regulatory documents related to TB, HIV, and AMR worldwide from 2015 through 2024
A comparative analysis of document categories revealed significant differences in the nature of policy documents across disease areas (Fig. 2). For HIV and tuberculosis (TB), a substantial proportion of documents comprised regulatory approvals and treatment guidance. Examples include U.S. Food and Drug Administration (FDA) supplemental new drug approvals, European Medicines Agency (EMA) opinions, and clinical guidelines, reflecting ongoing updates to therapeutic and diagnostic interventions for these diseases. Specifically, 35% of HIV-related documents were FDA supplemental new drug approvals (frequently concerning updates to antiretroviral indications), while 11% constituted press releases typically associated with such approvals. TB documents further encompassed regulatory actions, such as new TB drug approvals or orphan drug designations, which accounted for 17% of TB documents, alongside national TB program guidelines. In contrast, antimicrobial resistance (AMR)-related policy documents were predominantly strategic and collaborative in nature. The largest single category (28%) comprised records of multi-stakeholder meetings (e.g., One Health AMR coordination meetings and network reports), followed by policy reports, official communications, and governmental announcements of AMR action plans. Documents pertaining to product approvals were notably scarce within the AMR category. These patterns indicate that AMR policy efforts have primarily centered on strategic development and coordination, frequently within One Health frameworks, rather than regulating new therapeutics or establishing clinical guidelines.
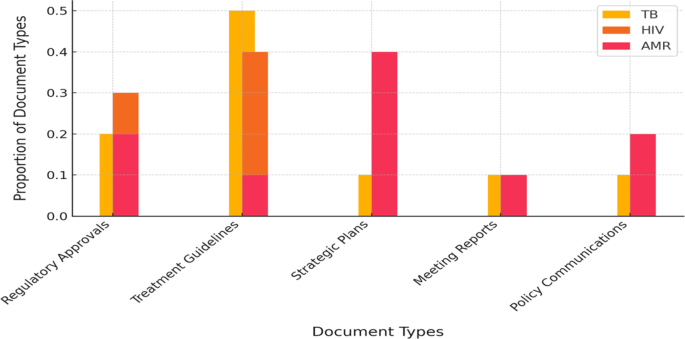
Distribution of document types for TB, HIV, and AMR policy documents (2015–2024)
The geographical scope of policy documents varied across the three domains. HIV-related policies issued between 2015 and 2024 encompassed 38 distinct jurisdictions, indicating extensive global engagement (including numerous high- and middle-income countries issuing HIV guidelines or regulatory approvals, alongside global/regional bodies). Tuberculosis (TB)-related documentation covered 29 countries/regions, demonstrating moderately reduced diversity yet maintaining a multi-continental distribution (predominantly initiated by the United States and European Union, with additional contributions from countries such as Thailand, China, South Africa, and Brazil). Antimicrobial resistance (AMR)-related policy instruments originated from merely 13 jurisdictions during the decade—primarily the European Union, United States, Canada, India, and a limited number of additional jurisdictions—reflecting the concentration of formal AMR policy initiatives (within the Cortellis-defined dataset) within specific regions. The EU and USA dominate across domains, acting as agenda-setting hubs.
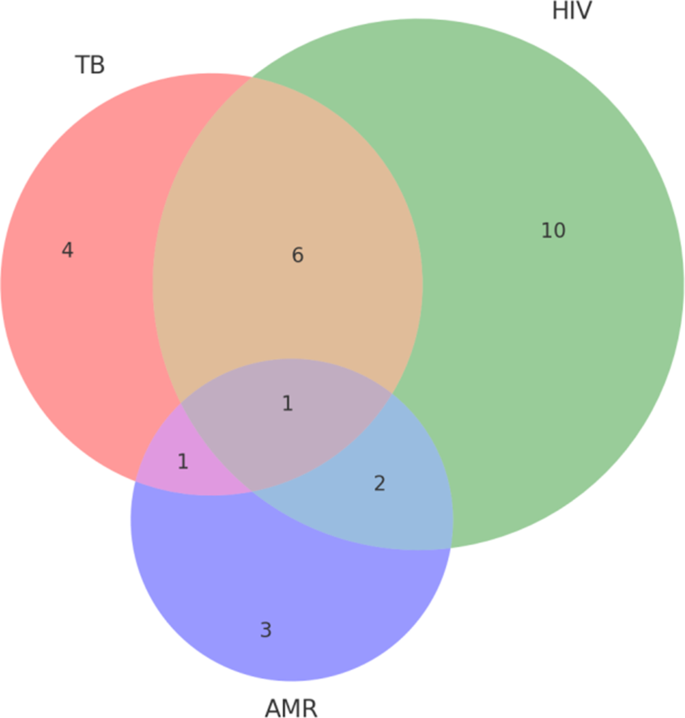
Overlap of jurisdictions involved in TB, HIV, and AMR policy documents (2015–2024)
A Venn analysis of jurisdictional coverage (Fig. 3) reveals that while a core group of jurisdictions (United States, European Union, India, China, South Korea, Kenya) have established policies addressing all three domains, policy efforts in numerous countries remain siloed. Notably, several countries (e.g., Russia, Greece, Philippines, Bulgaria) appeared exclusively within TB policy datasets, while others (e.g., Singapore, Chile, Switzerland, Australia) were documented solely within HIV policy datasets. Conversely, AMR policy activity was almost entirely confined to high-income settings (with jurisdictions such as Ireland, Sweden, and Germany appearing uniquely within AMR datasets). This distribution indicates that integrated policy attention to TB, HIV, and AMR collectively is predominantly confined to a limited number of jurisdictions, with most countries continuing to focus policy development on singular issues rather than adopting a combined approach.
The above quantitative findings underline that policy integration is not yet evident in the volume or breadth of global policy outputs – HIV has commanded the most policy attention, and AMR the least, with limited convergence. These data reinforce the need for greater integrated policy frameworks that can be adopted across more countries.
Qualitative results
Comparative analysis of coping strategies for TB, HIV, and AMR
The thematic framework analysis of the WHOs recent resolutions and decisions on tuberculosis, HIV, and antimicrobial resistance was detailed in Table 5 based on the social ecological model. The TB action plan focuses on improving coverage of treatment, rapid diagnostic tests, and increasing investment in TB research. This includes realigning strategies and targets at the country level, global collaboration, community engagement, and scaling up health services, especially for children and people with drug-resistant TB. The AIDS action plan focuses on strengthening national ownership of the health agenda, the role of community organizations, and WHOs role in strategic leadership, partnerships, and public health advocacy. It also highlights the use of primary health care platforms and the strategic integration of disease-specific and shared approaches based on country-specific contexts and health system capacities. The action plan on microbial resistance focuses on preventing all infections that can lead to antibiotic use, ensuring universal access to quality diagnosis and appropriate treatment, and enhancing strategic information and innovation. This involves the effective implementation of national action plans and the management of antibiotic use outside the health system. In summary, the action plans in all three areas emphasize leadership and ownership at the national level, global and regional cooperation, community engagement and the role of civil society organizations, and the importance of research and innovation. The difference is that the strategy for each disease area is tailored to its specific public health needs and challenges with targeted goals and approaches. Strategies for TB and HIV focus more on disease-specific treatment and prevention measures, while strategies for microbial resistance focus on cross-sectoral collaboration and the rational use of antimicrobials. In addition, monitoring and reporting mechanisms are used to ensure the effective implementation and continuous improvement of these action plans.
A thematic framework analysis based on the social-ecological model of the recent WHO governance and accountability mechanisms for TB, HIV, and antimicrobial resistance was detailed in Table 6. TB, AIDS, and microbial antimicrobial resistance demonstrate common governance principles and accountability mechanisms in governance and accountability strategies, including strategic leadership, partnership building, public health advocacy, norms and standards setting, technical support and capacity building, global monitoring and reporting, and the importance of funding. Nonetheless, strategies for each disease domain also set different disease-specific goals and implementation priorities based on their specific disease characteristics and global health challenges. For example, TB and AIDS may focus more on disease-specific treatment and prevention, while microbial resistance emphasizes cross-sectoral collaboration and rational use of anti-microbial agents. Furthermore, the focus of research and innovation, ways of community engagement, and specific implementation details of the accountability framework were also tailored to the global strategies and goals of each disease. The similarities and differences between these strategies reflect the flexibility and adaptability of global health in responding to different diseases while emphasizing the importance of cross-domain cooperation and common goals to ensure an effective response to global health challenges.
Action plans of stop TB, UNAIDS, and UNEP
Stop TB, UNAIDS, and UNEP, along with WHO, are the most influential international institutions in the field of tuberculosis, AIDS, and antimicrobial resistance (AMR) challenges. Their action plans and governance accountability mechanisms have the potential to impact the development of future resolutions by WHO. A comparative analysis of Stop TB, UNAIDS, and UNEP in their TB, AIDS, and AMR action plans is presented in Table 7. The action plans for tuberculosis, HIV, and antimicrobial resistance all highlight the significance of political leadership, legal and policy reforms, sufficient funding, community involvement, prevention and education efforts, treatment and care promotion, monitoring and evaluation systems, research and innovation, and international cooperation. These programs form a comprehensive public health response framework aimed at enhancing disease management, promoting health equity, reducing disease burden, and achieving global prevention goals.
Table 7 provides a comparative analysis of Stop TB, UNAIDS, and UNEP in their respective TB, AIDS, and antimicrobial resistance (AMR) action plans. The socio-ecological model-level comparative analysis summarized in Table 8 reveals commonalities and differences in governance and accountability for tuberculosis, HIV/AIDS, and microbial resistance (AMR). At the individual level, all three emphasized the importance of raising awareness, but each focused on specific implementation strategies. TB focused on health education, HIV focused on individual behavior change, and AMR focused on improving the knowledge of healthcare professionals and patients. At the interpersonal and community levels, all three focus on community engagement and support, as well as the strengthening of social networks. However, TB and HIV are more focused on community mobilization and communication between partners, while AMR emphasizes healthcare teams and doctor-patient communication. At the institutional level, all three action plans emphasize strengthening health systems and improving the quality of services, but AMR pays special attention to the development and implementation of antibiotic management policies. At the societal level, developing and implementing effective policies is a common goal, but TB and HIV are more focused on policy advocacy and funding allocation, while AMR focuses on policy and regulation development and the regulation of environmental emissions. Besides, all three stressed the importance of international cooperation, but AMR specifically mentioned the need to strengthen the involvement of the environmental sector and tackle emerging health threats with a One Health approach. Overall, while each disease has specific action plans and accountability mechanisms at different levels of the socio-ecological model, they all reflect the importance of cross-sectoral collaboration, community engagement, policy support and international cooperation in addressing public health challenges.
Integrated service for TB, HIV, and AMR
Funding for HIV/TB/AMR integration typically constitutes a subset of dedicated HIV, TB, or AMR budgets. For instance, major donors such as the Global Fund allocate substantial resources toward combating drug-resistant TB (DR-TB), exceeding US$2 billion during the 2020–2022 funding cycle for multidrug-resistant TB (MDR-TB) diagnosis and treatment within TB grants—directly contributing to AMR objectives. While the WHO End TB Strategy projected a requirement of approximately US$13 billion annually by 2022 for comprehensive TB programs, including MDR-TB, the actual available funding (only US$5–6 billion) signifies a critical underfunding gap for MDR-TB [61].
The AMR MPTFs country grants generally incorporate a TB component, such as strengthening TB laboratories for AMR surveillance. Consequently, a portion of this fund (projected to exceed US$70 million by 2024) supports TB-related AMR activities. Furthermore, research and development (R&D) funding for TB—encompassing novel antibiotics like bedaquiline and new vaccines—constitutes an integral component of the broader AMR investment landscape. Collectively, while TB represents a paramount priority within AMR strategic plans, dedicated funding remains inadequate, relying predominantly on general TB allocations supplemented by smaller AMR-specific grants.
Integrated HIV/TB projects have been instrumental in altering the trajectory of the TB/HIV co-epidemic. TB-associated mortality among people living with HIV (PLHIV) has declined significantly with the scale-up of integrated services. In 2023, 161,000 HIV-positive individuals died from TB, a substantial reduction from > over 400,000 annual deaths recorded in the mid-2000s [62]. This decline is largely attributable to the widespread implementation of antiretroviral therapy (ART) and isoniazid preventive therapy (IPT) within HIV care, coupled with routine HIV testing in TB care settings. Integration has yielded high service coverage: by 2018, 87% of TB patients in Africa knew their HIV status, and 86% of HIV-positive TB cases were receiving ART—a marked improvement from the approximately 55% testing coverage observed in 2015 [63, 64]. This linkage has demonstrably saved lives by ensuring timely treatment for both conditions in co-infected individuals. Global TB treatment success rates have consistently remained between 85 and 88% [61], indicating that integrating care for HIV-positive patients does not compromise treatment outcomes; rather, it enhances outcomes for co-infected individuals. Several countries (e.g., Eswatini, Rwanda) achieved significant reductions in AIDS-related mortality through the aggressive integration of TB/HIV services.
The integration of AMR-specific interventions into HIV and TB programs is comparatively recent; consequently, outcomes are primarily tracked in terms of systems enhancement and intermediate indicators. Key achievements include: the establishment of integrated AMR surveillance systems (encompassing HIV drug resistance, TB drug resistance, etc.), the development of One Health governance frameworks, and improved infection prevention and control (IPC) measures in healthcare facilities [65, 66]. By 2021, eight countries were actively implementing multisectoral AMR action plans supported by the AMR MPTF, thereby augmenting national capacities for detecting and responding to resistance threats [66].
TB/AMR integration focuses substantially on averting scenarios involving extreme economic burden. Treating a single MDR-TB patient can incur costs 10–20 times higher than treating a drug-susceptible TB case (exceeding US$10,000 per MDR-TB patient versus several hundred dollars for drug-susceptible TB in certain settings) [67]. Extensively drug-resistant TB (XDR-TB) entails even greater costs and is frequently associated with poor clinical outcomes. Therefore, preventing each case of MDR-TB—through effective initial treatment regimens and robust antimicrobial stewardship—represents significant cost savings. Integration facilitates prevention by rigorously applying directly observed therapy (DOT) to minimize acquired resistance and utilizing rapid diagnostics to expedite initiation of effective treatment. Moreover, integrating TB surveillance into broader AMR surveillance frameworks enhances cost-efficiency: infrastructure established to detect resistant TB can often be leveraged to identify other resistant infections, and vice versa. For example, integrated surveillance platforms (such as WHOs Tuberculosis Information System for Surveillance and Analysis, TISSA) capture TB drug resistance data alongside other AMR indicators [68]. To delineate distinctions between standalone disease programs and integrated initiatives, we summarizes key attributes of HIV, tuberculosis (TB), and antimicrobial resistance (AMR) initiatives from 2015 to 2024,details could be seen in Appendix Table 2 [64, 69,70,71,72]. Data and reports pertaining to HIV, TB, and AMR from the World Health Organization (WHO), the Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund), the Joint United Nations Programme on HIV/AIDS (UNAIDS), and the AMR Multi-Partner Trust Fund (AMR MPTF) are prioritized.
A consistent finding across studies is that integrated approaches yield superior outcomes compared to disease-specific programs in addressing HIV, tuberculosis (TB), and antimicrobial resistance (AMR), although certain implementation gaps persist [25, 73,74,75]. Comparative research conducted between 2015 and 2024 provides quantitative evidence substantiating the advantages of integrated healthcare strategies, with several countries demonstrating exemplary practices [73, 76, 77].
South Africa has emerged as a leader in implementing integrated HIV/TB care programs, which have demonstrated measurable reductions in co-infection rates [78,79,80]. These integrated programs deliver HIV and TB services concurrently by the same healthcare provider during a single clinical encounter [78]. A study in Durban, South Africa, documented the adoption of an integrated model at the primary healthcare level for HIV and TB service provision [78]. Furthermore, a quality improvement intervention in KwaZulu-Natal, South Africa, elucidated key lessons for enhancing integrated HIV-TB services [81]. The SUTHI trial in South Africa sought to reduce mortality through integrated TB and HIV services in rural primary healthcare clinics [75]. Research indicates that integrating TB management into HIV care significantly improves TB case detection and treatment initiation [79]. Mortality rates among patients with HIV and tuberculosis decreased following the implementation of integrated HIV-TB treatment [80]. However, operationalizing integrated services continues to present substantial challenges [82].
Uganda has also demonstrated success with TB-HIV integrated care. A long-term integrated TB-HIV care model at the Infectious Diseases Institute Clinic in Kampala, Uganda, evidenced sustained positive impacts on tuberculosis treatment outcomes [83]. Additionally, research in rural Ugandan health facilities established that TB/HIV integration enhances antiretroviral therapy (ART) initiation and TB treatment outcomes among TB/HIV-coinfected patients [84].
In Zambia, integrating HIV care and treatment into tuberculosis clinics in Lusaka improved TB/HIV care coordination and facilitated earlier ART initiation [74]. This integration specifically targeted diagnostic and treatment delays inherent to fragmented approaches [74]. An implementation research logic model underscores the critical elements of adaptability, design quality, and complexity in integrating HIV/NCD care in Lusaka [85].
India contends with a substantial burden of HIV and TB co-infection [86]. Integrated healthcare systems in India are conceptualized to address these concurrent epidemics, necessitating high-quality, community-based health systems [87]. Evidence suggests that integrating TB/HIV treatment improves service delivery and maximizes favorable treatment outcomes [88].
Massachusetts has integrated its public health response for HIV, viral hepatitis, sexually transmitted infections (STIs), and tuberculosis through policies mandating contracted organizations to submit specimens to the Massachusetts State Public Health Laboratory for testing [89].
Zimbabwe’s Population-based HIV Impact Assessment survey identified missed opportunities for TB diagnostic testing among people living with HIV (PLHIV), underscoring the imperative for enhanced service integration [90]. Among adult PLHIV engaged in HIV care, a significant proportion were not screened for TB during their most recent HIV care visit [90].
These diverse examples collectively illustrate that integrated approaches enhance health outcomes by effectively addressing co-infections and optimizing service delivery. Quality improvement interventions and the deployment of electronic medical records represent valuable strategies for strengthening the implementation of integrated care [81, 85]. While comprehensive cost-benefit analysis data of integration of TB,HIV and AMR is unavailable, we can infer that integrating TB, HIV, and AMR policies may lead to cost savings through shared infrastructure, reduced duplication of efforts, and improved health outcomes from joint implementation. Above analysis and case studies demonstrate that integrated service delivery can yield higher treatment success rates and more efficient use of resources.