This study aimed to compare the expression levels of TROP-2 and Nectin-4 in patients with upper tract urothelial carcinoma (UTUC) who had a prior history of urinary bladder carcinoma (UB-Ca) to those without such a history. While TROP-2 and Nectin-4 are well-studied therapeutic targets in UB-Ca, their comparative expression patterns in UTUC subgroups remain underexplored, particularly in patients with a history of UB-Ca. Our findings addressed this gap and highlighted critical differences with potential clinical implications.
We demonstrated that Nectin-4, the target protein of enfortumab vedotin, was expressed in 70.1% of patients with UTUC. This finding is consistent with the results of Tomiyama et al., who reported an expression rate of 65.7% in their cohort [10]. In UB-Ca, however, Nectin-4 expression has been documented in approximately 83% of cases, suggesting a slightly higher expression rate than UTUC [11]. Intriguingly, UTUC patients with prior UB-Ca history showed a non-significantly higher Nectin-4 expression rate (74.1% vs. 63.6%, p > 0.05). This trend mirrors findings by Klümper et al., who observed reduced Nectin-4 expression in metastatic urothelial carcinoma, potentially linked to EV resistance mechanisms [8]. While statistical significance was not achieved, this trend underscores the need for larger cohort studies to clarify the role of UB-Ca history in UTUC biology.
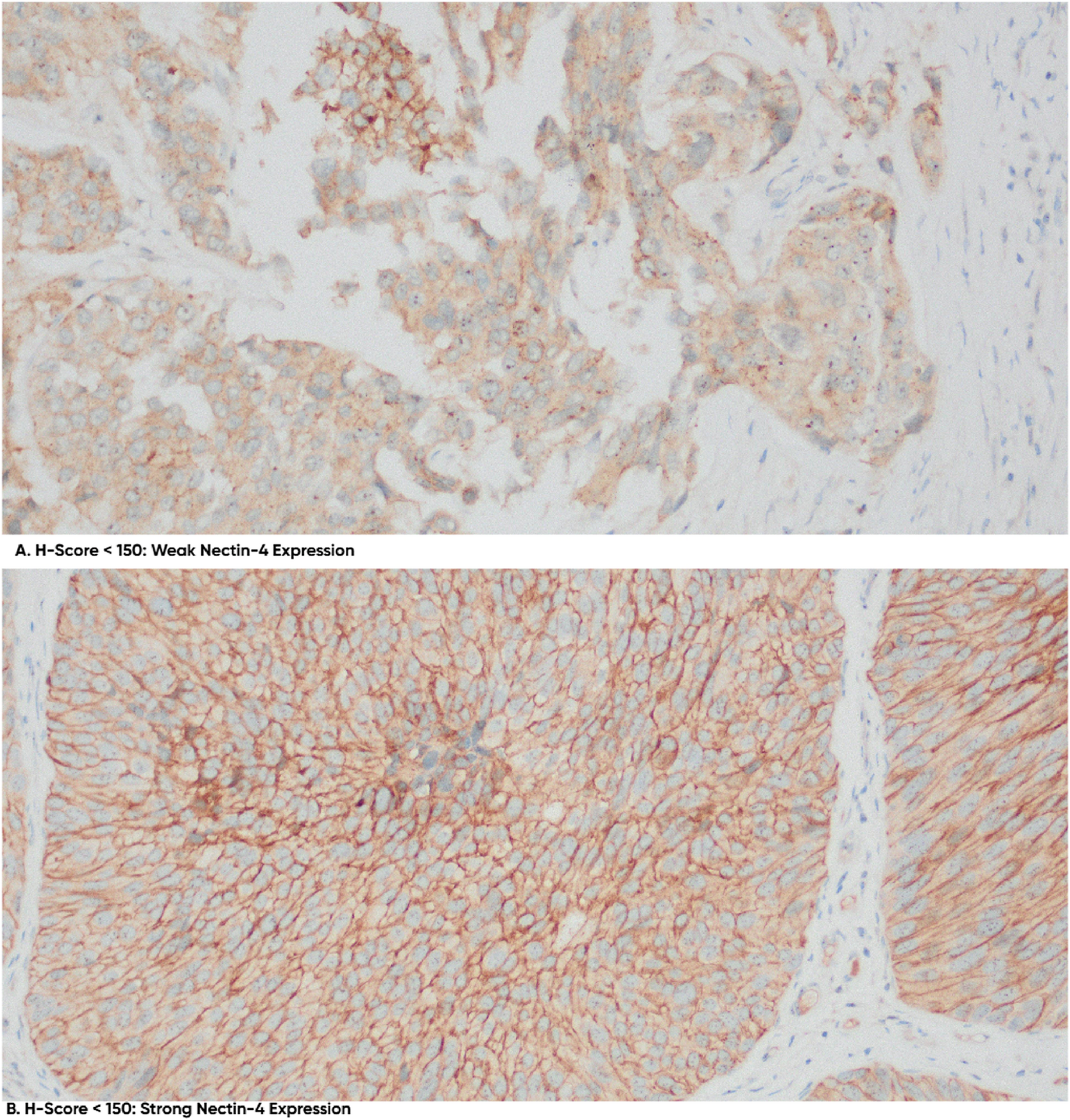
Powles et al. previously reported a median H-Score of 280 in patients with locally advanced or metastatic urothelial cancer, without distinguishing between UTUC and UB-Ca, in their exploratory analysis of Nectin-4 expression’s impact on outcomes with enfortumab vedotin (EV) plus pembrolizumab (P) in the Phase 3 EV-302 study [9]. Our cohort, however, showed markedly lower H-scores (mean 66), with 78.7% of patients falling into low-expression categories. This discrepancy may reflect both biological and methodological factors. Biologically, UTUC may inherently exhibit lower Nectin-4 expression due to its distinct urothelial differentiation like tumor stage (our cohort included localized UTUC vs. EV-302’s metastatic cases), immune context, or tumor evolution compared to bladder tumors [12,13,14]. Methodologically, variations in tissue fixation, antibody clones, or H-score evaluation across studies may contribute to the observed differences [13]. These findings emphasize the importance of tumor-site- and disease-stage-specific validation of Nectin-4 as a therapeutic biomarker before applying ADC-based treatment strategies. Despite the H-score widespread application, establishing standardized H-score thresholds for clinical responses remains a subject of debate, primarily due to variability in scoring systems across different institutions.
Recent studies have shown that TROP-2, the target protein of sacituzumab govitecan, is widely expressed in UTUC, with 94% of UTUC cases demonstrating positivity. High TROP-2 expression has also been associated with favorable prognosis in UTUC [15]. In our study, TROP-2 expression was detected in 98.8% (86 out of 87 patients), confirming the high expression rate of TROP-2 in UTUC, which is higher than that of Nectin-4. Notably, our findings were consistent with Tomiyama et al., who observed stronger TROP-2 expression (95.6%) in low-grade UTUC compared to high-grade variants, which was associated with a favorable prognosis [15]. This contrasts with findings in other cancers, such as non-muscle-invasive UB-Ca, breast cancer, and metastatic prostate cancer, where high TROP-2 expression has been linked to increased tumor aggressiveness and poor prognosis [15,16,17,18,19,20]. Recent UTUC studies indicate that high TROP-2 expression may instead reflect a more differentiated, luminal-like phenotype associated with favorable outcomes in this tumor type [15]. Differences in subcellular localization (membranous vs. cytoplasmic), signaling partners, and co-expression with luminal markers may modulate its function and prognostic implications in UTUC [15, 20]. In our cohort, no significant associations were found between TROP-2 expression and clinicopathological factors such as lymphovascular invasion or lymph node or distant metastasis.
Additionally, subgroup analysis based on UB-Ca classification did not reveal significant differences in Nectin-4 or TROP-2 expression. Although patients with a positive history of UB-Ca exhibited higher intensity of TROP-2 expression, the difference was not statistically significant. This suggests that a history of UB-Ca does not directly influence the expression levels of TROP-2 or Nectin-4 in UTUC.
The role of prior intravesical Bacillus Calmette–Guérin (BCG) treatment as a potential modifier of phenotypic marker expression, including Nectin‑4 and TROP‑2, remains understudied in UTUC. In non‑muscle‑invasive bladder cancer (NMIBC), multiple transcriptomic analyses have shown that both Nectin‑4 and TROP‑2 expression levels generally remain stable following BCG therapy, suggesting limited direct modulation by this immunotherapy [21]. However, another molecular study reported an upregulation of these markers post‑BCG exposure in a subset of tumors [22]. These conflicting findings may be influenced by tumor subtype, treatment duration, and intratumoral heterogeneity. In our retrospective UTUC cohort, detailed histories of bladder cancer treatment—including BCG or systemic chemotherapy—were available in only a minority of cases, precluding a robust subgroup analysis. We have therefore acknowledged this as an important limitation and potential confounding factor in interpreting biomarker expression in UTUC cases with prior bladder cancer history.
Our study has several limitations. First, its retrospective design limits causal inference, making it difficult to establish definitive relationships between TROP-2 and Nectin-4 expression and clinical outcomes. Second, the small and imbalanced sample size may limit statistical power. However, this reflects the rarity of UTUC itself—which comprises only 5–10% of urothelial tumors—and the even lower incidence of coexisting UTUC and prior UBC, which constrains prospective cohort size. Third, survival data were unavailable, preventing validation of the prognostic role of TROP-2 and Nectin-4. Fourth, potential prior treatments for UB-Ca, such as intravesical BCG or chemotherapy, may have affected expression of these markers, although such treatment histories were not systematically recorded. Future research should incorporate prospective, multi-institutional cohorts with standardized treatment documentation and centralized pathology review to improve generalizability and biomarker validation.