Triple-negative breast cancer (TNBC) is an aggressive subtype with limited treatment options and high relapse rates. The incorporation of immune checkpoint inhibitors (ICIs), particularly anti–PD-1/PD-L1 agents, into neoadjuvant chemotherapy (NAC) has improved outcomes for some patients. However, not all individuals benefit, and the risk of immune-related adverse events (irAEs) necessitates robust predictive biomarkers to guide treatment selection.
Less than 10% of patients experience meaningful benefit from the addition of ICIs to NAC. Hence, identifying biomarkers predictive of immunotherapy response remains an urgent priority.
Rationale for Tumor-Specific MHC-II (tsMHC-II)
Tumor-specific MHC class II expression has emerged as a promising immunotherapy biomarker in melanoma, bladder cancer, and now breast cancer. While MHC-II is traditionally associated with antigen-presenting cells, it can also be expressed on epithelial tumors, including breast cancers.
MHC-II molecules (e.g., HLA-DR) present exogenous antigens to CD4⁺ T cells, potentially activating adaptive immunity within the tumor microenvironment. The expression of these molecules on tumor cells (tsMHC-II) may signal an inflamed, immune-responsive tumor phenotype, making them especially relevant in the context of PD-L1 blockade.
NeoTRIPaPDL1 Trial design
The NeoTRIPaPDL1 trial was a phase III, multicenter, randomized, open-label study designed to test whether adding the PD-L1 inhibitor atezolizumab to neoadjuvant chemotherapy improves outcomes in high-risk early-stage triple-negative breast cancer. About 280 patients were assigned to receive eight cycles of carboplatin plus nab-paclitaxel, either alone or in combination with atezolizumab, followed by surgery and optional anthracycline chemotherapy.
The primary endpoint was event-free survival, with pathological complete response as a key secondary endpoint. To support translational research, baseline and on-treatment biopsies were collected and analyzed using advanced methods such as imaging mass cytometry, focusing on biomarkers like tumor-specific MHC-II. Conducted across multiple centers in Europe, Russia, and Asia, the trial combined rigorous clinical evaluation with biomarker discovery, aiming to refine patient selection for chemo-immunotherapy in triple-negative breast cancer.
NeoTRIPaPDL1 Trial Results
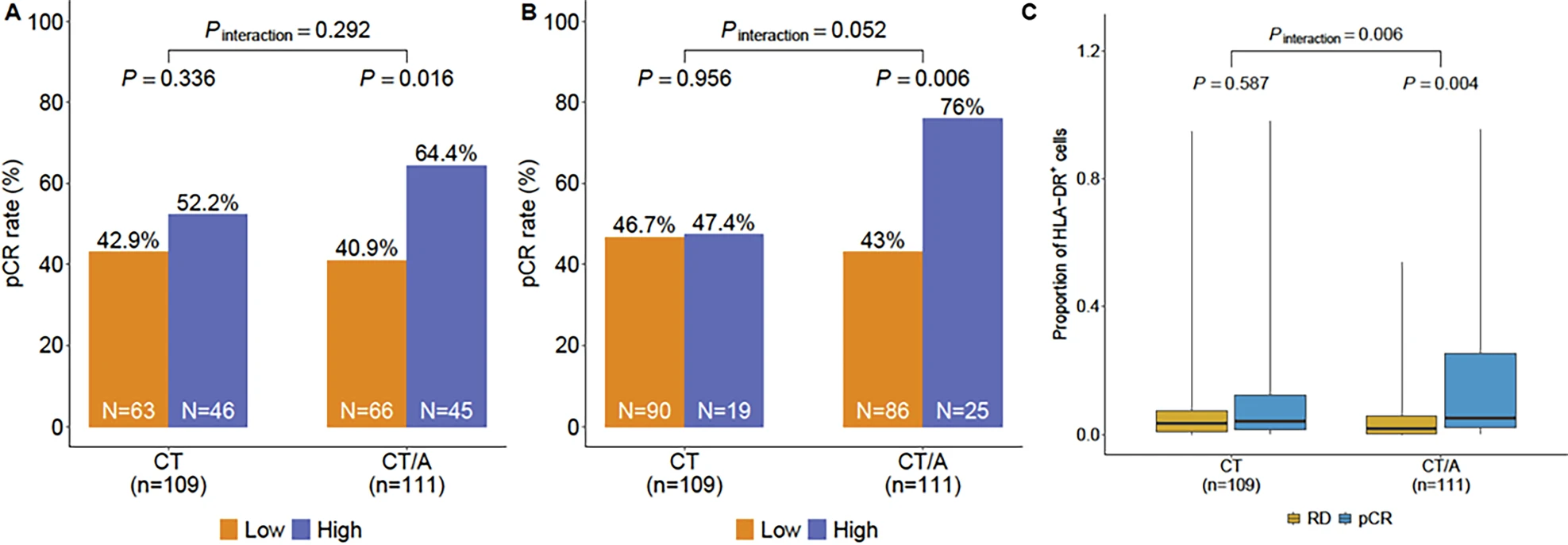
In the NeoTRIPaPDL1 trial, tumor-specific MHC-II expression was found in a substantial proportion of patients. Using the predefined 5% cutoff, 41% of triple-negative breast cancers were considered tsMHC-II positive, while application of a stricter exploratory cutoff identified positivity in about 20% of cases. The predictive value of this biomarker was most apparent in the chemo-immunotherapy arm. At the 5% cutoff, tsMHC-II positivity was associated with significantly higher pathological complete response rates, with an odds ratio of 2.58 and a p-value of 0.016. When the exploratory 80th percentile cutoff was applied, the association was even stronger, yielding an odds ratio of 4.19 and a p-value of 0.006.
In contrast, in the chemotherapy-only arm, tsMHC-II expression was not predictive of response. The odds ratio was 1.37 with a p-value of 0.34 at the 5% cutoff, and 1.03 with a p-value of 0.956 at the 80th percentile cutoff, indicating no meaningful relationship. Importantly, when tsMHC-II expression was evaluated as a continuous variable, a statistically significant interaction between biomarker status and treatment effect was observed, with a p-value of 0.006. Supporting these findings, receiver-operator characteristic analysis demonstrated improved predictive accuracy for pCR in the chemo-immunotherapy group compared to chemotherapy alone.
Together, these results underline the potential of tsMHC-II as a clinically relevant biomarker to identify patients most likely to benefit from the addition of atezolizumab to neoadjuvant chemotherapy in early-stage triple-negative breast cancer.
Insights and Discussion
- TsMHC-II consistently emerged as a predictive biomarker of ICI benefit in TNBC, unlike stromal TILs and PD-L1, which are largely prognostic or predictive of chemotherapy response.
- IMC provided greater sensitivity than traditional IHC or protein arrays, necessitating alternative cutoffs but confirming validity across methods.
- While the NeoTRIP regimen (atezolizumab + carboplatin/nab-paclitaxel) differs from the currently approved pembrolizumab-based NAC regimen, findings support tsMHC-II as a robust biomarker candidate.
- Limitations include lack of event-free survival (EFS) data and protocol-defined biomarker validation, making this an exploratory correlative analysis.
Key Takeaway Messages
- Tumor-specific MHC-II expression predicts pathological complete response specifically to chemo-immunotherapy, not chemotherapy alone, in early-stage TNBC.
- TsMHC-II outperforms PD-L1 and TILs as a predictive biomarker for immunotherapy benefit.
Ongoing Phase III trials (e.g., S1418, S2206) will further evaluate tsMHC-II in early breast cancer, with assay harmonization efforts underway. - If validated prospectively, tsMHC-II could enable personalized immunotherapy selection, sparing non-responders from unnecessary toxicity.
You Can Read All Article Here