In this case report, we highlight the dual-edged utility of [18F]FDG PET/CT in the management of breast cancer, illustrating both its strengths in staging and response assessment and its pitfalls related to specificity. The role of [18F]FDG PET/CT in breast cancer has been increasingly recognized, particularly for phenotypes characterized by high glycolytic activity—namely triple-negative and “HER2-enriched” subtypes [1, 5, 6]. The Phergain study prospectively demonstrated that changes in [18F]FDG uptake during neoadjuvant therapy can guide adaptive treatment strategies, improving pathological complete response rates in HER2-positive disease [7]. This study underscored the prognostic importance of metabolic response on interim PET and validated [18F]FDG PET/CT as a decision-making tool during treatment.
However, [18F]FDG PET/CT is not without limitations. Focal FDG-avid splenic lesions encompass a wide differential, including benign vascular entities such as cavernous or capillary hemangiomas, sclerosing angiomatoid nodular transformation (SANT), and less common vascular neoplasms like hemangioendothelioma [8, 9]. Other considerations include inflammatory or granulomatous disorders (e.g., sarcoidosis, tuberculosis, other mycobacterial or fungal infections), infectious abscess, hematologic involvement by lymphoma or leukemia, and metastatic disease. Several benign entities (notably SANT and atypical hemangiomas) can show variable FDG uptake and overlapping cross-sectional features, which complicate noninvasive diagnosis [10, 11]. Histopathology remains the reference standard for definitive diagnosis, but percutaneous splenic biopsy carries bleeding and sampling-error risks and may be impractical for small lesions—therefore, tailored integration of imaging features, clinical context, and multidisciplinary review is often required when tissue is not available.
A major drawback of [18F]FDG PET/CT is its limited specificity. [18F]FDG uptake is not tumor‐specific, and many benign processes—including inflammation, infection, and vascular lesions—can exhibit significant tracer accumulation. Large retrospective series and case reviews have similarly emphasized that splenic involvement by breast cancer is an uncommon event, with a pooled prevalence well below 1% across thousands of PET/CT studies [12, 13]. Consequently, the evidence of an FDG-avid splenic nodule in a breast cancer patient almost always prompts consideration of benign etiologies, with vascular anomalies such as hemangiomas ranking high on the differential.
In this context, MRI and contrast CT provide complementary morphologic and enhancement information to PET metabolic data. Typical cavernous hemangiomas usually appear markedly T2-hyperintense and demonstrate peripheral nodular enhancement with progressive centripetal fill-in on delayed phases (CT or MRI), while they may be variably FDG-avid [14]. SANT, although rare, often shows a characteristic spoke-wheel or star-shaped enhancement with a central fibrous scar and persistent delayed enhancement. In addition, on MRI, SANT may be heterogeneous with lower T2 signal centrally [15]. On PET/CT, both SANT and atypical vascular lesions can show increased [18F]FDG uptake, limiting specificity [10, 11]. Thus, enhancement pattern, diffusion behavior, and clinical context are the most useful MRI/CT discriminators, while PET adds sensitivity for metabolic activity but not reliable lesion-type specificity. In this regard, quantitative PET-derived parameters may help in distinguishing benign from malignant lesions. In particular, an SUV threshold of 2.3 has been reported to differentiate benign from malignant lesions, although this finding has not been validated in larger cohorts [8]. In our patient, in fact, the increased and focal tracer uptake (SUVmax 6.5) in the splenic lesion was misleading, as it suggested a neoplastic rather than a benign origin.
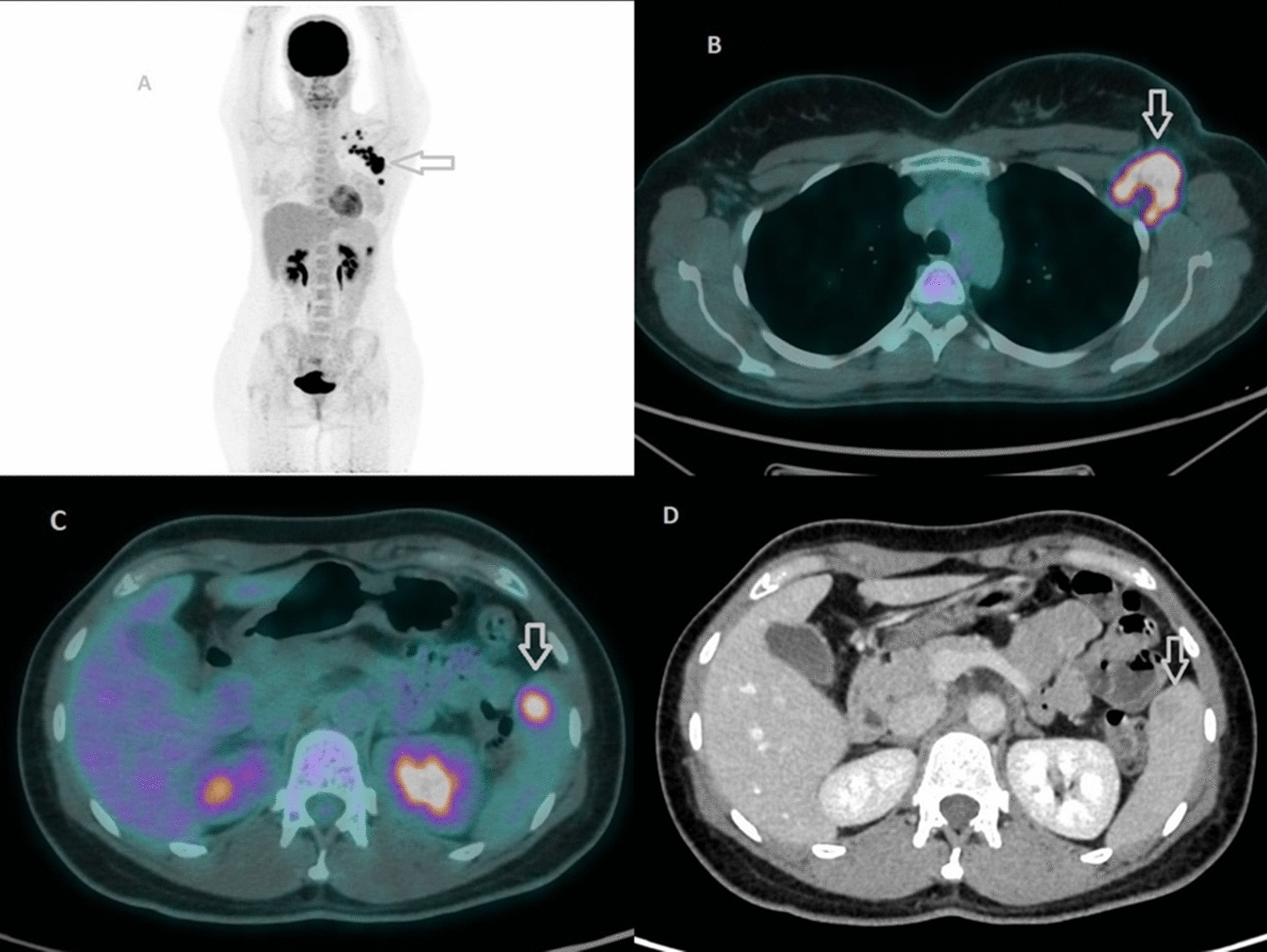
Our patient’s baseline staging PET/CT revealed an isolated hypermetabolic splenic lesion (SUVmax 6.5) with corresponding CT findings of a 16 mm hypodense nodule possessing a central low-density core and peripheral rim enhancement—features that are often worrisome for metastasis. Given the uncommon nature of splenic metastases in breast cancer, we pursued multimodal imaging. MRI characterization demonstrated no diffusion restriction and progressive contrast filling on delayed sequences, radiologically favoring an atypical hemangioma. Indeed, focal [18F]FDG uptake in atypical hemangiomas has been documented in both vertebral and hepatic locations, further complicating interpretation [16, 17]. In these reports, benign vascular tumors displayed intense [18F]FDG avidity akin to malignant lesions, likely reflecting the high endothelial cell turnover or inflammatory milieu within the lesion. What made our case particularly challenging was the combination of intense [18F]FDG uptake with CT features mimicking metastatic disease—a presentation that even experienced observers found disconcerting.
The therapeutic course provided an opportunity to further clarify the lesion’s nature. Following the first six cycles of neoadjuvant carboplatin, docetaxel, pertuzumab, and trastuzumab, repeat PET/CT demonstrated complete metabolic resolution of the splenic focus alongside reduction of the nodule to 5 mm on CT and preserved benign imaging characteristics on MRI. Such volume reduction of benign splenic lesions post‐therapy has been sporadically noted in the literature: one case series reported shrinkage of a splenic hemangioma following systemic chemotherapy for lymphoma [18]. However, to our knowledge, this is the first documented case of an atypical hemangioma in the spleen of a breast cancer patient evaluated in a true multimodal fashion—PET, CT, and MRI—both before and after neoadjuvant chemotherapy, with a demonstrated complete metabolic response.
The observed treatment-associated involution of the splenic lesion could be attributed to non‐specific effects of cytotoxic and targeted therapies on the vascular endothelium, inflammatory stroma, or associated macrophages within the hemangioma [19]. Such phenomena underscore the necessity of caution when interpreting post-therapy reductions in size or metabolic activity, as benign lesions may mimic true tumor response. In our patient, multidisciplinary review—bringing together nuclear medicine physicians, radiologists, oncologists, and surgeons—was pivotal in integrating imaging findings with clinical context, thereby avoiding overtreatment based on a false-positive PET result.
Despite these insights, several limitations must be acknowledged. First, the diagnosis of atypical hemangioma remains presumptive, as histologic confirmation was not available. Although percutaneous biopsy of small splenic lesions carries risks of hemorrhage and sampling error, tissue diagnosis remains the gold standard. Second, while [18F]FDG PET/CT remains widely accessible, its lack of specificity in differentiating benign from malignant vascular splenic lesions suggests a potential role for more selective radiotracers. Novel agents such as fibroblast activation protein inhibitor (FAPI) analogues have demonstrated low-background splenic uptake and high tumor-to-background ratios in breast cancer, offering promise for improved specificity [20, 21]. Preliminary studies of HER2-targeted agents labeled with positron emitters (e.g., 89Zr-trastuzumab) have also shown utility in assessing HER2 expression in metastatic lesions while sparing benign tissues [22]. These targeted tracers could potentially reduce false-positive findings in the spleen by exploiting receptor-based uptake rather than glycolytic activity alone.
Moreover, advanced MRI techniques—such as dynamic contrast-enhanced perfusion imaging, diffusion tensor imaging, and MR elastography—may provide additional tissue characterization metrics to distinguish benign vascular anomalies from metastatic deposits. Radiomics and machine learning approaches using multiparametric MRI and PET features may further refine diagnostic accuracy [23].