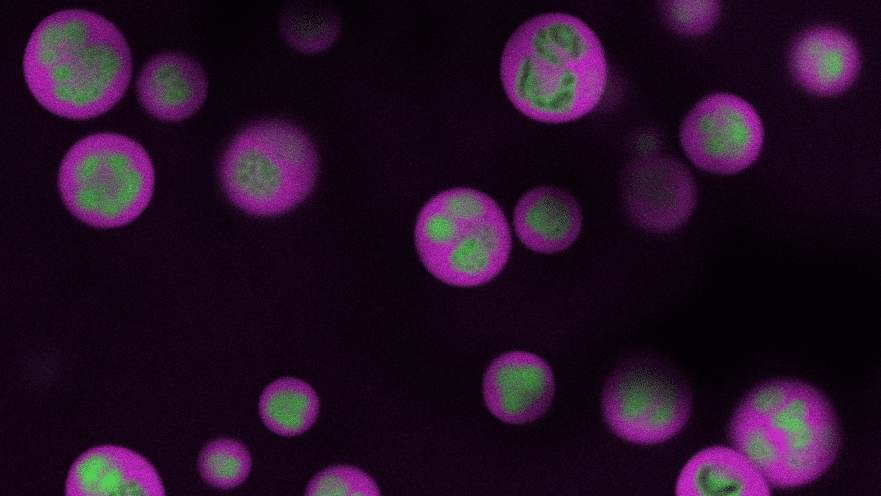
Proteins are the infinitely varied chemicals that make cells work, and science has a pretty good idea how they are made. But a critical aspect underlying the machinery of protein manufacture has long been hidden inside a blobby cellular structure called the nucleolus.
Now, a team of Princeton engineers have developed a technique to peer inside the nucleolus and reveal this hidden system of creation. Previous methods required researchers to break open the cell and destroy most of its structures, resulting in minimal access to the blob’s inner workings. By tracking the movement of RNA molecules inside the nucleolus using advanced imaging and genomics techniques, the new method allows researchers to watch these processes as they unfold without destroying the cell or its fragile components.
“These tools give us a window into what’s happening inside the nucleolus in a way we’ve never been able to see before,” said Clifford Brangwynne, the June K. Wu ’92 Professor of Chemical and Biological Engineering, the director of Princeton’s Omenn-Darling Bioengineering Institute, and the study’s principal investigator. “Now we have a precise spatial and temporal map,” he said.
Making an artificial nucleolus
The nucleolus is the largest structure inside the cell’s nucleus, key to cell growth and stress response. One of its main jobs is building ribosomes, which are the scaffolds that cells use to make proteins.
The team published details of the new method and an initial batch of findings that resulted from its use in the journal Nature on July 2.
In a first, the team also developed a way to make a simplified, artificial nucleolus. The model nucleoli allowed them to test ideas developed with the mapping technique and will play a complementary role in future experiments, according to the researchers.
Sofia Quinodoz, a postdoctoral fellow, and Lifei Jiang, a graduate student in molecular biology, spearheaded the work in Brangwynne’s lab.
These tools and other technologies developed in the Brangwynne lab were also highlighted in a recent article in Nature surveying the current state of this field.
What happens inside the nucleolus does not stay inside the nucleolus
The nucleolus itself is globular, with an inner, middle and outer layer. These layers consist of distinct liquid-like materials with physical differences — namely, surface tension — that keep them separated like oil and water. Each of these layers plays a different role in assembling the protein-making machines called ribosomes.
The researchers wanted to find a way to watch this ribosome-assembly process play out. Everything begins with RNA produced in the nucleolus’s innermost layer. That RNA assembles into components of what will become a new ribosome. As the components move through the layers of the nucleolus, they are assembled in a stepwise fashion to form ribosomes. With the mapping technique, Quinodoz and Jiang track this process in detail, from the initial formation of components to the finished product.
“This is exciting because we previously didn’t know how the layers are built,” Quinodoz said.
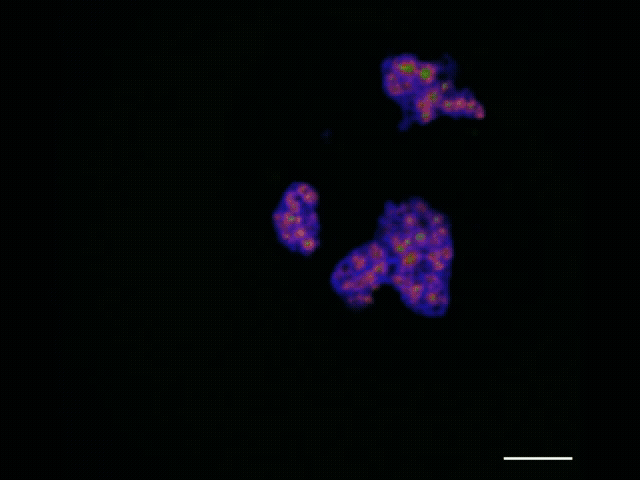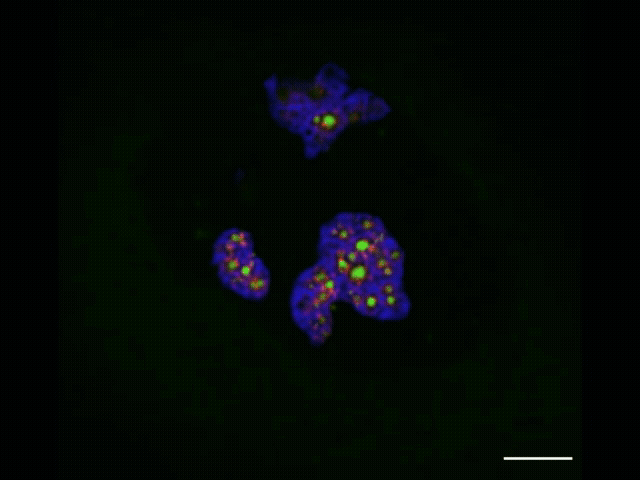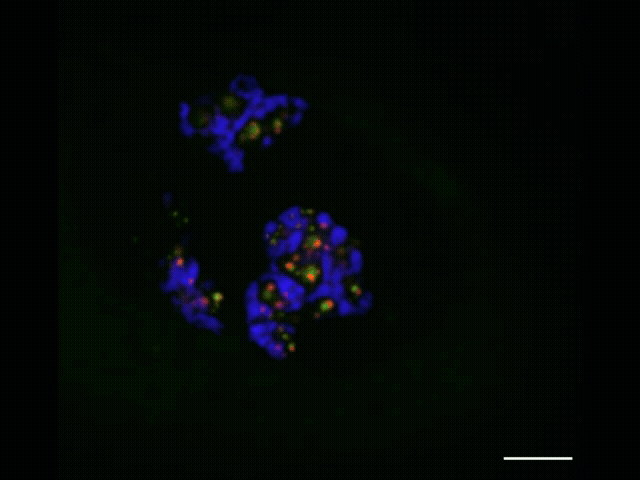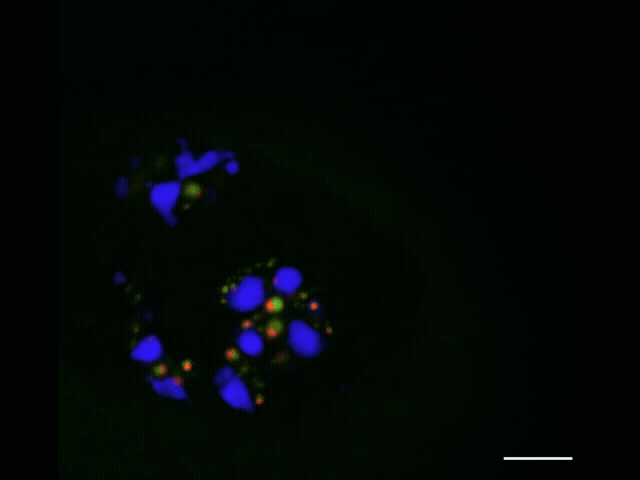
To watch the assembly process play out, she and Jiang applied advanced sequencing and imaging techniques, capturing snapshots as RNA moved along the assembly line that allowed them to track the movements of each part. Along with advanced microscopy techniques, they observed that properly processed ribosomal RNA moves through the nucleolus from inner to middle to outer layer and then leaves the nucleolus, and that specific assembly steps to the ribosomal RNA occur inside each layer. As this was unfolding, they observed that the ribosome’s smaller subunit is mostly assembled in the inner and middle layers while the larger subunit is assembled throughout all three layers.
Interestingly, they found that disrupting these processes created major problems with the structure of the nucleolus. In one test, RNA accumulated within the middle layer and not in the outer layer, prompting the outer layer to detach from the middle layer and form a kind of necklace around the smaller sphere. Another test resulted in the nucleolus turning itself inside-out, reversing the order of the layers. Working with Princeton colleagues including Andrej Košmrlj, associate professor of mechanical and aerospace engineering, graduate student Qiwei Yu, and former Princeton Bioengineering Institute Innovators Fellow Hongbo Zhao, the group was able to show how the perturbations to RNA processing alter surface tension, to drive this inside-out structuring.
“We got all these hints that the structure is being built around the RNA, and its processing is shaping the structure, making it turn inside out or fall apart when its normal function is disrupted,” said Quinodoz, a Hanna Gray Fellow at the Howard Hughes Medical Institute (HHMI) and a 2013 Princeton alumna.
Quality control checkpoints
The Princeton group teamed up with ribosome experts Denis Lafontaine of Université libre de Bruxelles and Sebastian Klinge of Rockefeller University, to disrupt different steps in the ribosome assembly line and use a system called a DNA plasmid to induce living cells to create brand new, human-designed nucleoli. They found that the synthesized structures functioned much like the natural ones, with the larger ribosome subunits assembling more slowly than the smaller ones. They were also able to replicate the inside-out structures that they saw in the defective nucleoli. By manipulating the RNA and causing the nucleolus to react accordingly, they identified a key feature that can be studied in greater detail.
“We uncovered that this complex factory in the cell has essential quality control checkpoints,” said Quinodoz. “The ribosomal RNA is moving from one part of the factory to another only if the processing step is actually done. Then it releases into the next step.”
Now that they have these tools, Brangwynne and his group are looking at what happens in diseases like cancer, where more ribosomes are produced in cancerous cells than in healthy cells. Using their mapping tool, they hope to find vulnerabilities in the production process which could be targets for therapeutics. “Nobody has really mapped that in detail yet,” said Jiang.
The paper “Mapping and engineering RNA-driven architecture of the multiphase nucleolus” was published July 2, 2025 in Nature. In addition to Brangwynne, Quinodoz, Jiang, Zhao, Yu, Košmrlj, Lafontaine and Klinge, the authors included Aya A. Abu-Alfa, Troy J. Comi, Lennard W. Wiesner, Jordy F. Botello, Anita Đonlić and Elizabeth Soehalim of Princeton University; Prashant Bhat of the California Institute of Technology and the University of California-Los Angeles; and Christiane Zorbas and Ludivine Wacheul of Université libre de Bruxelles. Support for this project was provided by HHMI, the National Science Foundation, the St. Jude Medical Foundation, Princeton University, the Chan Zuckerberg Initiative Exploratory Network, the Princeton Biomolecular Condensate Program, the Princeton Center for Complex Materials (NSF MRSEC, DMR-2011750), the Princeton University Office of Undergraduate Research, W. Reid Pitts Jr. Senior Thesis Fund in Molecular Biology/Biology, the Eleanor A. Crecca Senior Thesis Research Fund for Molecular Biology, Princeton Bioengineering Institute Innovators (PBI2) Postdoctoral Fellowship, Princeton University Harold W. Dodds Fellowship, Chen Graduate Innovator Grant, Josephine De Karman Fellowship Trust, European Cooperation in Science and Technology (COST), Fonds De La Recherche Scientifique – FNRS, EOS [CD-INFLADIS 40007512] Région Wallonne (SPW EER) Win4SpinOff [RIBOGENESIS] European Joint Programme on Rare Diseases (EJP-RD) RiboEurope DBAGene Cure, U.S. Department of Health and Human Services | National Institutes of Health, and the G. Harold and Leila Y. Mathers Foundation.