Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–36.
Google Scholar
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Google Scholar
Jokhadze N, Das A, Dizon DS. Global cancer statistics: A healthy population relies on population health. CA Cancer J Clin. 2024;74:224–6.
Google Scholar
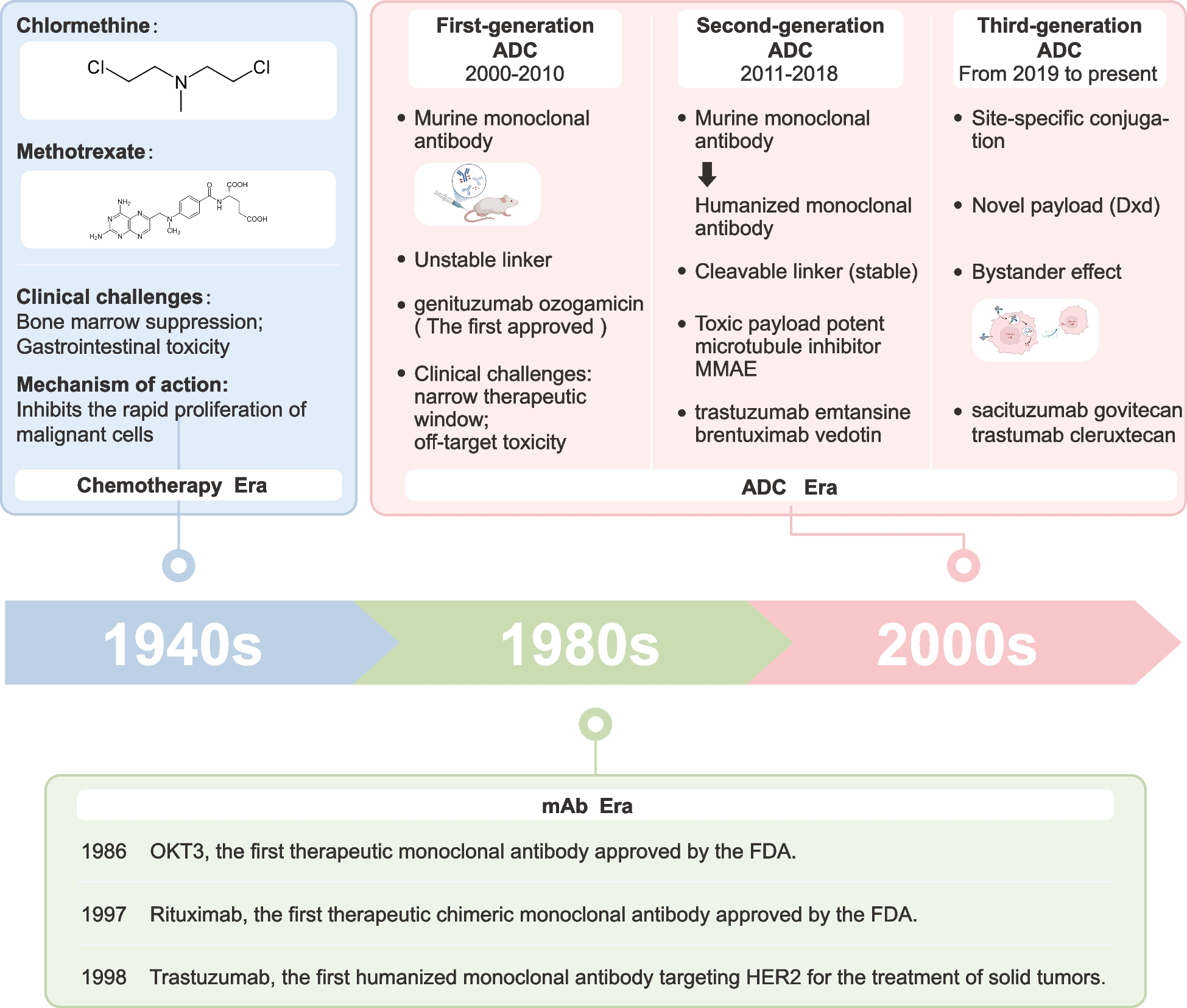
Chabner BA, Roberts TG Jr. Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72.
Google Scholar
DeVita VT Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–53.
Google Scholar
Jang JY, Kim D, Kim ND. Recent Developments in Combination Chemotherapy for Colorectal and Breast Cancers with Topoisomerase Inhibitors. Int J Mol Sci. 2023;24:8457.
Tilsed CM, Fisher SA, Nowak AK, Lake RA, Lesterhuis WJ. Cancer chemotherapy: insights into cellular and tumor microenvironmental mechanisms of action. Front Oncol. 2022;12:960317.
Google Scholar
Liu D, Auguste DT. Cancer targeted therapeutics: From molecules to drug delivery vehicles. J Control Release. 2015;219:632–43.
Google Scholar
Tibau A, Hwang TJ, Molto C, Avorn J, Kesselheim AS. Clinical value of molecular targets and FDA-approved genome-targeted cancer therapies. JAMA Oncol. 2024;10:634–41.
Google Scholar
Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8.
Google Scholar
Finn RS. Development of molecularly targeted therapies in hepatocellular carcinoma: where do we go now? Clin Cancer Res. 2010;16:390–7.
Google Scholar
Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395:1078–88.
Google Scholar
Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51.
Google Scholar
Tabish TA, Narayan RJ. Mitochondria-targeted graphene for advanced cancer therapeutics. Acta Biomater. 2021;129:43–56.
Google Scholar
Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7:93.
Google Scholar
Tsuchikama K, Anami Y, Ha SYY, Yamazaki CM. Exploring the next generation of antibody-drug conjugates. Nat Rev Clin Oncol. 2024;21:203–23.
Google Scholar
Tolcher AW. Antibody drug conjugates: lessons from 20 years of clinical experience. Ann Oncol. 2016;27:2168–72.
Google Scholar
Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9:33–46.
Google Scholar
Theocharopoulos C, Lialios PP, Samarkos M, Gogas H, Ziogas DC. Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond. Vaccines (Basel). 2021;9:1111.
Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16:315–37.
Google Scholar
Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35:e00225.
Matikonda SS, McLaughlin R, Shrestha P, Lipshultz C, Schnermann MJ. Structure-Activity Relationships of Antibody-Drug Conjugates: A Systematic Review of Chemistry on the Trastuzumab Scaffold. Bioconjug Chem. 2022;33:1241–53.
Google Scholar
Zhao P, Zhang Y, Li W, Jeanty C, Xiang G, Dong Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm Sin B. 2020;10:1589–600.
Google Scholar
Gogia P, Ashraf H, Bhasin S, Xu Y. Antibody-Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence. Cancers (Basel). 2023;15:3886.
Gauzy-Lazo L, Sassoon I, Brun MP. Advances in Antibody-Drug Conjugate Design: Current Clinical Landscape and Future Innovations. SLAS Discov. 2020;25:843–68.
Google Scholar
Ruan DY, Wu HX, Meng Q, Xu RH. Development of antibody-drug conjugates in cancer: Overview and prospects. Cancer Commun (Lond). 2024;44:3–22.
Google Scholar
Alrosan AZ, Dmour I, Rataan AO, Heilat GB, Alrosan K. Comparative pharmacological analysis of fam-trastuzumab deruxtecan-nxki and sacituzumab govitecan-hziy: Two recently developed chemotherapies in the crucial battle against breast cancer. Toxicol Rep. 2025;14:102054.
Google Scholar
Gandullo-Sánchez L, Ocaña A, Pandiella A. Generation of Antibody-Drug Conjugate Resistant Models. Cancers (Basel). 2021;13:4631.
Khoury R, Saleh K, Khalife N, Saleh M, Chahine C, Ibrahim R, et al. Mechanisms of Resistance to Antibody-Drug Conjugates. Int J Mol Sci. 2023;24:9674.
Hunter FW, Barker HR, Lipert B, Rothé F, Gebhart G, Piccart-Gebhart MJ, et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br J Cancer. 2020;122:603–12.
Google Scholar
Ma J, Chan JJ, Toh CH, Yap YS. Emerging systemic therapy options beyond CDK4/6 inhibitors for hormone receptor-positive HER2-negative advanced breast cancer. NPJ Breast Cancer. 2023;9:74.
Google Scholar
Birnboim-Perach R, Benhar I. Using combination therapy to overcome diverse challenges of immune checkpoint inhibitors treatment. Int J Biol Sci. 2024;20:3911–22.
Google Scholar
Teicher BA, Morris J. Antibody-drug conjugate targets, drugs, and linkers. Curr Cancer Drug Targets. 2022;22:463–529.
Google Scholar
Larrosa C, Mora J, Cheung NK. Global Impact of Monoclonal Antibodies (mAbs) in Children: A Focus on Anti-GD2. Cancers (Basel). 2023;15:3729.
Zhang X, Huang AC, Chen F, Chen H, Li L, Kong N, et al. Novel development strategies and challenges for anti-Her2 antibody-drug conjugates. Antib Ther. 2022;5:18–29.
Google Scholar
Huang H, Zhou Y, Shang C, Zhang Y, Shen Y. A novel anti-HER2/EGFR bispecific antibody-drug conjugate demonstrates promising antitumor efficacy and overcomes resistance to HER2- or EGFR-targeted ADCs. Invest New Drugs. 2025;43:262–75.
Google Scholar
Yamazaki CM, Yamaguchi A, Anami Y, Xiong W, Otani Y, Lee J, et al. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun. 2021;12:3528.
Google Scholar
Yue H, Xu H, Ma L, Li X, Yang B, Wang X, et al. A DXd/TLR7-Agonist Dual-Conjugate Anti-HER2 ADC Exerts Robust Antitumor Activity Through Tumor Cell Killing and Immune Activation. Mol Cancer Ther. 2024;23:1639–51.
Google Scholar
Zhao RJ, Fan XX. Advances in Antibody-Based Immune-Stimulating Drugs: Driving Innovation in Cancer Therapy. Int J Mol Sci. 2025;26:1440.
Zhang C, Liu Y, Li G, Yang Z, Han C, Sun X, et al. Targeting the undruggables-the power of protein degraders. Sci Bull (Beijing). 2024;69:1776–97.
Google Scholar
Kang DH, Lee J, Im S, Chung C. Navigating the Complexity of Resistance in Lung Cancer Therapy: Mechanisms, Organoid Models, and Strategies for Overcoming Treatment Failure. Cancers (Basel). 2024;16:3996.
Bandara S, Raveendran S. Current Landscape and Future Directions in Cancer Immunotherapy: Therapies, Trials, and Challenges. Cancers (Basel). 2025;17:821.
Tarantino P, Carmagnani Pestana R, Corti C, Modi S, Bardia A, Tolaney SM, et al. Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72:165–82.
Google Scholar
Fuentes-Antrás J, Genta S, Vijenthira A, Siu LL. Antibody-drug conjugates: in search of partners of choice. Trends Cancer. 2023;9:339–54.
Google Scholar
Conilh L, Sadilkova L, Viricel W, Dumontet C. Payload diversification: a key step in the development of antibody-drug conjugates. J Hematol Oncol. 2023;16:3.
Google Scholar
Shen BQ, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30:184–9.
Google Scholar
Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18:327–44.
Google Scholar
Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet. 2019;394:793–804.
Google Scholar
Ponziani S, Di Vittorio G, Pitari G, Cimini AM, Ardini M, Gentile R, et al. Antibody-Drug Conjugates: The New Frontier of Chemotherapy. Int J Mol Sci. 2020;21:5510.
Khera E, Thurber GM. Pharmacokinetic and Immunological Considerations for Expanding the Therapeutic Window of Next-Generation Antibody-Drug Conjugates. BioDrugs. 2018;32:465–80.
Google Scholar
Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023;22:101–26.
Google Scholar
Indini A, Rijavec E, Grossi F. Trastuzumab Deruxtecan: Changing the Destiny of HER2 Expressing Solid Tumors. Int J Mol Sci. 2021;22:4774.
Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16:888–97.
Google Scholar
Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–65.
Google Scholar
Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022;29:1335–44.
Google Scholar
Liu K, Li M, Li Y, Li Y, Chen Z, Tang Y, et al. A review of the clinical efficacy of FDA-approved antibody-drug conjugates in human cancers. Mol Cancer. 2024;23:62.
Google Scholar
Rugo HS, Bardia A, Marmé F, Cortés J, Schmid P, Loirat D, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402:1423–33.
Google Scholar
Shastry M, Jacob S, Rugo HS, Hamilton E. Antibody-drug conjugates targeting TROP-2: clinical development in metastatic breast cancer. Breast. 2022;66:169–77.
Google Scholar
Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, et al. A Biparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell. 2016;29:117–29.
Google Scholar
Perez Bay AE, Faulkner D, DaSilva JO, Young TM, Yang K, Giurleo JT, et al. A Bispecific METxMET Antibody-Drug Conjugate with Cleavable Linker Is Processed in Recycling and Late Endosomes. Mol Cancer Ther. 2023;22:357–70.
Google Scholar
Evers A, Krah S, Demir D, Gaa R, Elter D, Schroeter C, et al. Engineering hydrophobicity and manufacturability for optimized biparatopic antibody-drug conjugates targeting c-MET. MAbs. 2024;16:2302386.
Google Scholar
Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26:925–32.
Google Scholar
Zhou Q. Site-Specific Antibody Conjugation with Payloads beyond Cytotoxins. Molecules. 2023;28:917.
Gu Y, Zhao Q. Clinical Progresses and Challenges of Bispecific Antibodies for the Treatment of Solid Tumors. Mol Diagn Ther. 2024;28:669–702.
Google Scholar
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–31.
Google Scholar
Qiao G, Kone LB, Phillips EH, Lee SS, Brown GE, Khetani SR, et al. LIGHT enhanced bispecific antibody armed T-cells to treat immunotherapy resistant colon cancer. Oncogene. 2022;41:2054–68.
Google Scholar
Singh A, Dees S, Grewal IS. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer. 2021;124:1037–48.
Google Scholar
Gu Y, Wang Z, Wang Y. Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharm Sin B. 2024;14:1965–86.
Google Scholar
Wei B, Gunzner-Toste J, Yao H, Wang T, Wang J, Xu Z, et al. Discovery of Peptidomimetic Antibody-Drug Conjugate Linkers with Enhanced Protease Specificity. J Med Chem. 2018;61:989–1000.
Google Scholar
Mahmood I. Clinical Pharmacology of Antibody-Drug Conjugates. Antibodies (Basel). 2021;10:20.
Paci A, Desnoyer A, Delahousse J, Blondel L, Maritaz C, Chaput N, et al. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 1, monoclonal antibodies, antibody-drug conjugates and bispecific T-cell engagers. Eur J Cancer. 2020;128:107–18.
Google Scholar
Bargh JD, Isidro-Llobet A, Parker JS, Spring DR. Cleavable linkers in antibody-drug conjugates. Chem Soc Rev. 2019;48:4361–74.
Google Scholar
Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody-Drug Conjugates: A Comprehensive Review. Mol Cancer Res. 2020;18:3–19.
Google Scholar
Su Z, Xiao D, Xie F, Liu L, Wang Y, Fan S, et al. Antibody-drug conjugates: recent advances in linker chemistry. Acta Pharm Sin B. 2021;11:3889–907.
Google Scholar
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90.
Google Scholar
Dorywalska M, Strop P, Melton-Witt JA, Hasa-Moreno A, Farias SE, Galindo Casas M, et al. Effect of attachment site on stability of cleavable antibody drug conjugates. Bioconjug Chem. 2015;26:650–9.
Google Scholar
Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 2010;21:5–13.
Google Scholar
Chari RV, Miller ML, Widdison WC. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed Engl. 2014;53:3796–827.
Google Scholar
Agarwal P, Bertozzi CR. Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug Chem. 2015;26:176–92.
Google Scholar
Pillow TH, Sadowsky JD, Zhang D, Yu SF, Del Rosario G, Xu K, et al. Decoupling stability and release in disulfide bonds with antibody-small molecule conjugates. Chem Sci. 2017;8:366–70.
Google Scholar
Kellogg BA, Garrett L, Kovtun Y, Lai KC, Leece B, Miller M, et al. Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjug Chem. 2011;22:717–27.
Google Scholar
Erickson HK, Widdison WC, Mayo MF, Whiteman K, Audette C, Wilhelm SD, et al. Tumor delivery and in vivo processing of disulfide-linked and thioether-linked antibody-maytansinoid conjugates. Bioconjug Chem. 2010;21:84–92.
Google Scholar
Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13:47–58.
Google Scholar
Feld J, Barta SK, Schinke C, Braunschweig I, Zhou Y, Verma AK. Linked-in: design and efficacy of antibody drug conjugates in oncology. Oncotarget. 2013;4:397–412.
Google Scholar
Hafeez U, Parakh S, Gan HK, Scott AM. Antibody-Drug Conjugates for Cancer Therapy. Molecules. 2020;25:4764.
Hinman LM, Hamann PR, Wallace R, Menendez AT, Durr FE, Upeslacis J. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res. 1993;53:3336–42.
Google Scholar
Lambert JM, Chari RV. Ado-trastuzumab emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem. 2014;57:6949–64.
Google Scholar
Sanchez-Moreno P, Ortega-Vinuesa JL, Peula-Garcia JM, Marchal JA, Boulaiz H. Smart drug-delivery systems for cancer nanotherapy. Curr Drug Targets. 2018;19:339–59.
Google Scholar
Martínez-Carmona M, Lozano D, Colilla M, Vallet-Regí M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018;65:393–404.
Google Scholar
Teicher BA, Chari RV. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17:6389–97.
Google Scholar
Hobson AD. Antibody drug conjugates beyond cytotoxic payloads. Prog Med Chem. 2023;62:1–59.
Google Scholar
Gromek SM, Balunas MJ. Natural products as exquisitely potent cytotoxic payloads for antibody- drug conjugates. Curr Top Med Chem. 2015;14:2822–34.
Google Scholar
Shefet-Carasso L, Benhar I. Antibody-targeted drugs and drug resistance–challenges and solutions. Drug Resist Updat. 2015;18:36–46.
Google Scholar
Singh D, Dheer D, Samykutty A, Shankar R. Antibody drug conjugates in gastrointestinal cancer: from lab to clinical development. J Control Release. 2021;340:1–34.
Google Scholar
Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019;111:538–49.
Google Scholar
Jiang J, Li S, Tang N, Wang L, Xin W, Li S. Preclinical safety profile of RC88-ADC: a novel mesothelin-targeted antibody conjugated with Monomethyl auristatin E. Drug Chem Toxicol. 2023;46:24–34.
Google Scholar
Bianchi G, Anderson KC. Understanding biology to tackle the disease: Multiple myeloma from bench to bedside, and back. CA Cancer J Clin. 2014;64:422–44.
Google Scholar
Almodovar Diaz AA, Alouch SS, Chawla Y, Gonsalves WI. The antibody drug conjugate, belantamab-mafodotin, in the treatment of multiple myeloma: a comprehensive review. Blood Lymphat Cancer. 2024;14:71–87.
Google Scholar
Scribner JA, Hicks SW, Sinkevicius KW, Yoder NC, Diedrich G, Brown JG, et al. Preclinical Evaluation of IMGC936, a Next-Generation Maytansinoid-based Antibody-drug Conjugate Targeting ADAM9-expressing Tumors. Mol Cancer Ther. 2022;21:1047–59.
Google Scholar
Marei HE, Cenciarelli C, Hasan A. Potential of antibody-drug conjugates (ADCs) for cancer therapy. Cancer Cell Int. 2022;22:255.
Google Scholar
Wiedemeyer WR, Gavrilyuk J, Schammel A, Zhao X, Sarvaiya H, Pysz M, et al. ABBV-011, A Novel, Calicheamicin-Based Antibody-Drug Conjugate, Targets SEZ6 to Eradicate Small Cell Lung Cancer Tumors. Mol Cancer Ther. 2022;21:986–98.
Google Scholar
Yao HP, Zhao H, Hudson R, Tong XM, Wang MH. Duocarmycin-based antibody-drug conjugates as an emerging biotherapeutic entity for targeted cancer therapy: pharmaceutical strategy and clinical progress. Drug Discov Today. 2021;26:1857–74.
Google Scholar
Joseph AM, Nahar K, Daw S, Hasan MM, Lo R, Le TBK, et al. Mechanistic insight into the repair of C8-linked pyrrolobenzodiazepine monomer-mediated DNA damage. RSC Med Chem. 2022;13:1621–33.
Google Scholar
Anderson MG, Zhang Q, Rodriguez LE, Hecquet CM, Donawho CK, Ansell PJ, et al. ABBV-176, a PRLR antibody drug conjugate with a potent DNA-damaging PBD cytotoxin and enhanced activity with PARP inhibition. BMC Cancer. 2021;21:681.
Google Scholar
Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, et al. Antibody-drug conjugates: current status and future directions. Drug Discov Today. 2014;19:869–81.
Google Scholar
Khera E, Dong S, Huang H, de Bever L, van Delft FL, Thurber GM. Cellular-Resolution Imaging of Bystander Payload Tissue Penetration from Antibody-Drug Conjugates. Mol Cancer Ther. 2022;21:310–21.
Google Scholar
Wang R, Hu B, Pan Z, Mo C, Zhao X, Liu G, et al. Antibody-Drug Conjugates (ADCs): current and future biopharmaceuticals. J Hematol Oncol. 2025;18:51.
Google Scholar
Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. MAbs. 2014;6:34–45.
Google Scholar
Izzo D, Ascione L, Guidi L, Marsicano RM, Koukoutzeli C, Trapani D, et al. Innovative payloads for ADCs in cancer treatment: moving beyond the selective delivery of chemotherapy. Ther Adv Med Oncol. 2025;17:17588359241309460.
Google Scholar
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387:9–20.
Google Scholar
Rosellini M, Santoni M, Mollica V, Rizzo A, Cimadamore A, Scarpelli M, et al. Treating Prostate Cancer by Antibody-Drug Conjugates. Int J Mol Sci. 2021;22:1551.
Bardia A, Sun S, Thimmiah N, Coates JT, Wu B, Abelman RO, et al. Antibody-drug conjugate sacituzumab govitecan enables a sequential TOP1/PARP inhibitor therapy strategy in patients with breast cancer. Clin Cancer Res. 2024;30:2917–24.
Google Scholar
Najdi T, Awad L, Chartouni A, Soueidy C, Kourie H. Navigating antibody‒drug conjugates (ADCs): from metastatic to early breast cancer treatment strategies. Invest New Drugs. 2025;43:466-503.
Cheung A, Chenoweth AM, Johansson A, Laddach R, Guppy N, Trendell J, et al. Anti-EGFR antibody-drug conjugate carrying an inhibitor targeting CDK restricts triple-negative breast cancer growth. Clin Cancer Res. 2024;30:3298–315.
Google Scholar
Boni V, Sharma MR, Patnaik A. The Resurgence of Antibody Drug Conjugates in Cancer Therapeutics: Novel Targets and Payloads. Am Soc Clin Oncol Educ Book. 2020;40:1–17.
Google Scholar
Umotoy JC, de Taeye SW. Antibody Conjugates for Targeted Therapy Against HIV-1 as an Emerging Tool for HIV-1 Cure. Front Immunol. 2021;12:708806.
Google Scholar
Choi J, Jang H, Choi J, Choi Y, Yang Y, Shim MK, et al. Immune checkpoint-targeted drug conjugates: a promising tool for remodeling tumor immune microenvironment. J Control Release. 2023;359:85–96.
Google Scholar
Li C, Shi K, Zhao S, Liu J, Zhai Q, Hou X, et al. Natural-source payloads used in the conjugated drugs architecture for cancer therapy: Recent advances and future directions. Pharmacol Res. 2024;207:107341.
Google Scholar
Xi M, Zhu J, Zhang F, Shen H, Chen J, Xiao Z, et al. Antibody-drug conjugates for targeted cancer therapy: recent advances in potential payloads. Eur J Med Chem. 2024;276:116709.
Google Scholar
Riccardi F, Dal Bo M, Macor P, Toffoli G. A comprehensive overview on antibody-drug conjugates: from the conceptualization to cancer therapy. Front Pharmacol. 2023;14:1274088.
Google Scholar
Kovtun YV, Goldmacher VS. Cell killing by antibody-drug conjugates. Cancer Lett. 2007;255:232–40.
Google Scholar
Nguyen TD, Bordeau BM, Balthasar JP. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers (Basel). 2023;15:713.
Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov. 2023;22:641–61.
Google Scholar
Colombo R, Tarantino P, Rich JR, LoRusso PM, de Vries EGE. The Journey of Antibody-Drug Conjugates: Lessons Learned from 40 Years of Development. Cancer Discov. 2024;14:2089–108.
Google Scholar
Okada R, Asakage T. Near-infrared photoimmunotherapy: basics and clinical application. Jpn J Clin Oncol. 2025;55:843-851.
Buyukgolcigezli I, Tenekeci AK, Sahin IH. Opportunities and Challenges in Antibody-Drug Conjugates for Cancer Therapy: A New Era for Cancer Treatment. Cancers (Basel). 2025;17:958.
Lv Y, Cui X, Li T, Liu C, Wang A, Wang T, et al. Mechanism of action and future perspectives of ADCs in combination with immune checkpoint inhibitors for solid tumors. Clin Exp Med. 2025;25:139.
Google Scholar
Jen EY, Ko CW, Lee JE, Del Valle PL, Aydanian A, Jewell C, et al. FDA Approval: Gemtuzumab Ozogamicin for the Treatment of Adults with Newly Diagnosed CD33-Positive Acute Myeloid Leukemia. Clin Cancer Res. 2018;24:3242–6.
Google Scholar
Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–6.
Google Scholar
Chia CSB. A Patent Review on FDA-Approved Antibody-Drug Conjugates. Their Linkers and Drug Payloads ChemMedChem. 2022;17:e202200032.
Google Scholar
Jiang X, Nik Nabil WN, Ze Y, Dai R, Xi Z, Xu H. Unlocking natural potential: antibody-drug conjugates with naturally derived payloads for cancer therapy. Phytother Res. 2025;39:789–874.
Google Scholar
Salinas Y, Chauhan SC, Bandyopadhyay D. Small-Molecule Mitotic Inhibitors as Anticancer Agents: Discovery, Classification, Mechanisms of Action, and Clinical Trials. Int J Mol Sci. 2025;26:3279.
Narayan P, Dilawari A, Osgood C, Feng Z, Bloomquist E, Pierce WF, et al. US food and drug administration approval summary: Fam-trastuzumab deruxtecan-nxki for human epidermal growth factor receptor 2-low unresectable or metastatic breast cancer. J Clin Oncol. 2023;41:2108–16.
Google Scholar
Spring LM, Nakajima E, Hutchinson J, Viscosi E, Blouin G, Weekes C, et al. Sacituzumab govitecan for metastatic triple-negative breast cancer: clinical overview and management of potential toxicities. Oncologist. 2021;26:827–34.
Google Scholar
Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab Govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–41.
Google Scholar
Samantasinghar A, Sunildutt NP, Ahmed F, Soomro AM, Salih ARC, Parihar P, et al. A comprehensive review of key factors affecting the efficacy of antibody drug conjugate. Biomed Pharmacother. 2023;161:114408.
Google Scholar
Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386:241–51.
Google Scholar
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–30.
Google Scholar
Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Durán I, Lee JL, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384:1125–35.
Google Scholar
Johns AC, Campbell MT. Toxicities From Antibody-Drug Conjugates. Cancer J. 2022;28:469–78.
Google Scholar
D’Arienzo A, Verrazzo A, Pagliuca M, Napolitano F, Parola S, Viggiani M, et al. Toxicity profile of antibody-drug conjugates in breast cancer: practical considerations. EClinicalMedicine. 2023;62:102113.
Google Scholar
Pedersini R, Buffoni M, Petrelli F, Ghidini A, di Mauro P, Amoroso V, et al. Gastrointestinal toxicity of antibody drug conjugates (ADCs) in metastatic breast cancer: a pooled analysis. Clin Breast Cancer. 2024;24:411–20.
Google Scholar
Atiq S, Hirshman N, Shariff A, Zhang T. The management of toxicities from immune, targeted and ADCs treatments in patients with urothelial cancer. Urol Oncol. 2023;41:410–9.
Google Scholar
Shi Y, Yao K, Zhao J, Yue Y, Wu H. Gastrointestinal toxicity of antibody-drug conjugates: a pharmacovigilance study using the FAERS database. BMC Pharmacol Toxicol. 2025;26:50.
Google Scholar
Baba T, Kusumoto M, Kato T, Kurihara Y, Sasaki S, Oikado K, et al. Clinical and imaging features of interstitial lung disease in cancer patients treated with trastuzumab deruxtecan. Int J Clin Oncol. 2023;28:1585–96.
Google Scholar
Liao D, Zhang J, Yan T, Chen Y, Fu Y, Xie N, et al. A systematic review of mechanisms, incidence, and management of Trastuzumab deruxtecan induced ILD/pneumonitis in solid tumors. Drug Des Devel Ther. 2025;19:1655–68.
Google Scholar
Tsao LC, Wang JS, Ma X, Sodhi S, Ragusa JV, Liu B, et al. Effective extracellular payload release and immunomodulatory interactions govern the therapeutic effect of trastuzumab deruxtecan (T-DXd). Nat Commun. 2025;16:3167.
Google Scholar
Rugo HS, Tolaney SM, Loirat D, Punie K, Bardia A, Hurvitz SA, et al. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer. 2022;8:98.
Google Scholar
Best RL, LaPointe NE, Azarenko O, Miller H, Genualdi C, Chih S, et al. Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): mechanistic insights into MMAE ADC peripheral neuropathy. Toxicol Appl Pharmacol. 2021;421:115534.
Google Scholar
Lacouture ME, Patel AB, Rosenberg JE, O’Donnell PH. Management of dermatologic events associated with the Nectin-4-directed antibody-drug conjugate Enfortumab Vedotin. Oncologist. 2022;27:e223–32.
Google Scholar
Bouleftour W, Guillot A, Magne N. The anti-Nectin 4: a promising tumor cells target. A systematic review. Mol Cancer Ther. 2022;21:493–501.
Google Scholar
Metrangolo V, Engelholm LH. Antibody-Drug Conjugates: The Dynamic Evolution from Conventional to Next-Generation Constructs. Cancers (Basel). 2024;16:447.
Sasso JM, Tenchov R, Bird R, Iyer KA, Ralhan K, Rodriguez Y, et al. The evolving landscape of antibody-drug conjugates. In depth analysis of recent research progress. Bioconjug Chem. 2023;34:1951–2000.
Google Scholar
Bogani G, Coleman RL, Vergote I, van Gorp T, Ray-Coquard I, Oaknin A, et al. Mirvetuximab soravtansine-gynx: first antibody/antigen-drug conjugate (ADC) in advanced or recurrent ovarian cancer. Int J Gynecol Cancer. 2024;34:469–77.
Google Scholar
Yao P, Zhang Y, Zhang S, Wei X, Liu Y, Du C, et al. Knowledge atlas of antibody-drug conjugates on CiteSpace and clinical trial visualization analysis. Front Oncol. 2022;12:1039882.
Google Scholar
Krop IE, Masuda N, Mukohara T, Takahashi S, Nakayama T, Inoue K, et al. Patritumab deruxtecan (HER3-DXd), a human epidermal growth factor receptor 3-directed antibody-drug conjugate, in patients with previously treated human epidermal growth factor receptor 3-expressing metastatic breast cancer: a multicenter, phase I/II trial. J Clin Oncol. 2023;41:5550–60.
Google Scholar
Feustel K, Martin J, Falchook GS. B7–H3 inhibitors in oncology clinical trials: a review. J Immunother Precis Oncol. 2024;7:53–66.
Google Scholar
Brignole C, Calarco E, Bensa V, Giusto E, Perri P, Ciampi E, et al. Antitumor activity of the investigational B7-H3 antibody-drug conjugate, vobramitamab duocarmazine, in preclinical models of neuroblastoma. J Immunother Cancer. 2023;11:e007174.
Decary S, Berne PF, Nicolazzi C, Lefebvre AM, Dabdoubi T, Cameron B, et al. Preclinical activity of SAR408701: a novel Anti-CEACAM5-maytansinoid antibody-drug conjugate for the treatment of CEACAM5-positive epithelial tumors. Clin Cancer Res. 2020;26:6589–99.
Google Scholar
Cardenas KCA, Enos CW, Spear MR, Austin DE, Almofeez R, Kortchak S, et al. CT109-SN-38, a novel antibody-drug conjugate with dual specificity for CEACAM5 and 6, elicits potent killing of pancreatic cancer cells. Curr Cancer Drug Targets. 2024;24:720–32.
Google Scholar
Xie M, Li P, Lu H, Dong S, Wilski N, Pandey P, et al. Abstract 2880: TM4SF1 as a novel target for different modalities (mAb, bispecific ADC, targeted siRNA) for the treatment of different solid tumor cancers. Cancer Res. 2025;85:2880–2880.
Visintin A, Knowlton K, Tyminski E, Lin CI, Zheng X, Marquette K, et al. Novel Anti-TM4SF1 Antibody-Drug Conjugates with Activity against Tumor Cells and Tumor Vasculature. Mol Cancer Ther. 2015;14:1868–76.
Google Scholar
Abrams T, Connor A, Fanton C, Cohen SB, Huber T, Miller K, et al. Preclinical antitumor activity of a novel anti-c-KIT antibody-drug conjugate against mutant and wild-type c-KIT-positive solid tumors. Clin Cancer Res. 2018;24:4297–308.
Google Scholar
Kim C, Cho JG, Wi T, Moon J, Ko H-J, Lee J, et al. Abstract 3142: NN3201, a novel c-Kit targeting ADC, exhibits robust preclinical anti-tumor efficacy in SCLC and GIST models. Cancer Res. 2024;84:3142–3142.
Gitto SB, Whicker M, Davies G, Kumar S, Kinneer K, Xu H, et al. A B7-H4-targeting antibody-drug conjugate shows antitumor activity in PARPi and platinum-resistant cancers with B7–H4 expression. Clin Cancer Res. 2024;30:1567–81.
Google Scholar
Kinneer K, Wortmann P, Cooper ZA, Dickinson NJ, Masterson L, Cailleau T, et al. Design and preclinical evaluation of a novel B7-H4-directed antibody-drug conjugate, AZD8205, alone and in combination with the PARP1-selective inhibitor AZD5305. Clin Cancer Res. 2023;29:1086–101.
Google Scholar
Jänne PA, Baik C, Su WC, Johnson ML, Hayashi H, Nishio M, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov. 2022;12:74–89.
Google Scholar
Tabernero J, Bedard PL, Bang YJ, Vieito M, Ryu MH, Fagniez N, et al. Tusamitamab ravtansine in patients with advanced solid tumors: phase I study of safety, pharmacokinetics, and antitumor activity using alternative dosing regimens. Cancer Res Commun. 2023;3:1662–71.
Google Scholar
Gazzah A, Bedard PL, Hierro C, Kang YK, Abdul Razak A, Ryu MH, et al. Safety, pharmacokinetics, and antitumor activity of the anti-CEACAM5-DM4 antibody-drug conjugate tusamitamab ravtansine (SAR408701) in patients with advanced solid tumors: first-in-human dose-escalation study. Ann Oncol. 2022;33:416–25.
Google Scholar
Bardia A, Krop IE, Kogawa T, Juric D, Tolcher AW, Hamilton EP, et al. Datopotamab deruxtecan in advanced or metastatic HR+/HER2- and triple-negative breast cancer: results from the phase I TROPION-PanTumor01 study. J Clin Oncol. 2024;42(19):2281–94.
Google Scholar
Shatsky RA, Trivedi MS, Yau C, Nanda R, Rugo HS, Davidian M, et al. Datopotamab-deruxtecan plus durvalumab in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial. Nat Med. 2024;30:3737–47.
Google Scholar
Scribner JA, Brown JG, Son T, Chiechi M, Li P, Sharma S, et al. Preclinical development of MGC018, a duocarmycin-based antibody-drug conjugate targeting B7–H3 for solid cancer. Mol Cancer Ther. 2020;19:2235–44.
Google Scholar
Malapelle U, Parente P, Pepe F, Di Micco MC, Russo A, Clemente C, et al. B7-H3/CD276 Inhibitors: Is There Room for the Treatment of Metastatic Non-Small Cell Lung Cancer? Int J Mol Sci. 2022;23:16077.
Turner N, Saura C, Aftimos P, van den Tweel E, Oesterholt M, Koper N, et al. Trastuzumab Duocarmazine in Pretreated Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Breast Cancer: An Open-Label, Randomized, Phase III Trial (TULIP). J Clin Oncol. 2025;43:513–23.
Google Scholar
Raghav KPS, Hubert A, Fakih M, Fang H, Aristide MRN, Almeida CBd, et al. Phase 2 randomized study evaluating safety, efficacy, and optimal dose of ABBV-400 in combination with fluorouracil, folinic acid, and bevacizumab in previously treated patients with metastatic colorectal cancer. J Clin Oncol. 2024;42:TPS3636-TPS3636.
Ma Y, Huang Y, Zhao Y, Zhao S, Xue J, Yang Y, et al. BL-B01D1, a first-in-class EGFR-HER3 bispecific antibody-drug conjugate, in patients with locally advanced or metastatic solid tumours: a first-in-human, open-label, multicentre, phase 1 study. Lancet Oncol. 2024;25:901–11.
Google Scholar
Zhou ZZ, Si Y, Zhang J, Chen K, George A, Kim S, et al. A dual-payload antibody-drug conjugate targeting CD276/B7-H3 elicits cytotoxicity and immune activation in triple-negative breast cancer. Cancer Res. 2024;84:3848–63.
Google Scholar
Xiao D, Liu L, Xie F, Dong J, Wang Y, Xu X, et al. Azobenzene-based linker strategy for selective activation of antibody-drug conjugates. Angew Chem Int Ed Engl. 2024;63:e202310318.
Google Scholar
Hamadani M, Collins GP, Caimi PF, Samaniego F, Spira A, Davies A, et al. Camidanlumab tesirine in patients with relapsed or refractory lymphoma: a phase 1, open-label, multicentre, dose-escalation, dose-expansion study. Lancet Haematol. 2021;8:e433–45.
Google Scholar
Kahl BS, Hamadani M, Radford J, Carlo-Stella C, Caimi P, Reid E, et al. A phase I study of ADCT-402 (loncastuximab tesirine), a novel pyrrolobenzodiazepine-based antibody-drug conjugate, in relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2019;25:6986–94.
Google Scholar
Collins GP, Horwitz SM, Davies A, Karnad A, Samaniego F, Spira AI, et al. Adct-301 (Camidanlumab Tesirine), a novel pyrrolobenzodiazepine-based CD25-targeting antibody drug conjugate, in a phase 1 study of relapsed/refractory non-Hodgkin lymphoma shows activity in T-cell lymphoma. Blood. 2018;132:1658.
Zhou C, Wang B, Teng C, Yang H, Piha-Paul SA, Richardson G, et al. A phase Ia study of a novel anti-HER2 antibody-drug conjugate GQ1001 in patients with previously treated HER2 positive advanced solid tumors. J Transl Med. 2025;23:37.
Google Scholar
Saleh K, Khoury R, Khalife N, Chahine C, Ibrahim R, Tikriti Z, et al. Mechanisms of action and resistance to anti-HER2 antibody-drug conjugates in breast cancer. Cancer Drug Resist. 2024;7:22.
Google Scholar
Gupta R, Kumar R, Penn CA, Wajapeyee N. Immune evasion in ovarian cancer: implications for immunotherapy and emerging treatments. Trends Immunol. 2025;46:166–81.
Google Scholar
Zhao H, Xu Z, Li C, Xu T, Zhang J, Jiao J, et al. Efficacy and safety of Disitamab Vedotin combined with programmed death-1 inhibitor for advanced urothelial cancer: a case-series study. Adv Ther. 2024;41:857–66.
Google Scholar
He L, Wang L, Wang Z, Li T, Chen H, Zhang Y, et al. Immune modulating antibody-drug conjugate (IM-ADC) for cancer immunotherapy. J Med Chem. 2021;64:15716–26.
Google Scholar
Shi X, Tang K, Zhang Q, Han Q, Quan L, Li Y, et al. Antibody-drug conjugate combinations in cancer treatment: clinical efficacy and clinical study perspectives. Front Pharmacol. 2025;16:1556245.
Google Scholar
Dong S, Li X, Huang Q, Li Y, Li J, Zhu X, et al. Resistance to immunotherapy in non-small cell lung cancer: unraveling causes, developing effective strategies, and exploring potential breakthroughs. Drug Resist Updat. 2025;81:101215.
Google Scholar
Wang G, Yang H, Wang Y, Qin J. Ovarian cancer targeted therapy: current landscape and future challenges. Front Oncol. 2025;15:1535235.
Google Scholar
Lalli G, Sabatucci I, Paderno M, Martinelli F, Signorelli M, Maruccio M, et al. Navigating the landscape of resistance mechanisms in antibody-drug conjugates for cancer treatment. Target Oncol. 2025;20:419–30.
Google Scholar
Jose J, Hooper JD, Souza-Fonseca-Guimaraes F. Highlights of 2024. Broadening anti-cancer immunotherapy modalities with antibody-drug conjugates: emerging insights from clinical studies. Immunol Cell Biol. 2025;103:530-534.
Tahayneh K, Idkedek M, Abu Akar F. NSCLC: Current Evidence on Its Pathogenesis, Integrated Treatment, and Future Perspectives. J Clin Med. 2025;14:1025.
Ouyang W, Xu Z, Guan S, Hu Y, Gou X, Liu Z, et al. Advancement opportunities and endeavor of innovative targeted therapies for small cell lung cancer. Int J Biol Sci. 2025;21:1322–41.
Google Scholar
Bragasin EI, Cheng J, Ford L, Poei D, Ali S, Hsu R. Advances in adoptive cell therapies in small cell lung cancer. Exploration of Targeted Anti-tumor Therapy. 2025;6:1002302.
Google Scholar
Xie D, Wang Q, Wu G. Research progress in inducing immunogenic cell death of tumor cells. Front Immunol. 2022;13:1017400.
Google Scholar
Tan X, Fang P, Li K, You M, Cao Y, Xu H, et al. A HER2-targeted antibody-novel DNA topoisomerase I inhibitor conjugate induces durable adaptive antitumor immunity by activating dendritic cells. MAbs. 2023;15:2220466.
Google Scholar
Müller P, Martin K, Theurich S, Schreiner J, Savic S, Terszowski G, et al. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol Res. 2014;2:741–55.
Google Scholar
Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med. 2019;23:4854–65.
Google Scholar
Schmid P, Loi S, De la Cruz Merino L, Yerushalmi R, Im SA, Sonnenblick A, et al. 181O Interim analysis (IA) of the atezolizumab (atezo) + sacituzumab govitecan (SG) arm in patients (pts) with triple-negative breast cancer (TNBC) in MORPHEUS-pan BC: A phase Ib/II study of multiple treatment (tx) combinations in pts with locally advanced/metastatic BC (LA/mBC). ESMO Open. 2024;9:109203
Malli Cetinbas N, Monnell T, Soomer-James J, Shaw P, Lancaster K, Catcott KC, et al. Tumor cell-directed STING agonist antibody-drug conjugates induce type III interferons and anti-tumor innate immune responses. Nat Commun. 2024;15:5842.
Google Scholar
Long R, Zuo H, Tang G, Zhang C, Yue X, Yang J, et al. Antibody-drug conjugates in cancer therapy: applications and future advances. Front Immunol. 2025;16:1516419.
Google Scholar
Bhatnagar S, Revuri V, Shah M, Larson P, Shao Z, Yu D, et al. Combination of STING and TLR 7/8 Agonists as Vaccine Adjuvants for Cancer Immunotherapy. Cancers (Basel). 2022;14:6091.
Camidge DR, Barlesi F, Goldman JW, Morgensztern D, Heist R, Vokes E, et al. Phase Ib study of telisotuzumab vedotin in combination with erlotinib in patients with c-Met protein-expressing non-small-cell lung cancer. J Clin Oncol. 2023;41(5):1105–15.
Google Scholar
Kujtan L, Subramanian J. Telisotuzumab vedotin with erlotinib in the treatment of non-small cell lung cancer: a well MET combination? Transl Lung Cancer Res. 2023;12:1826–9.
Google Scholar
Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390:875–88.
Google Scholar
Hanna KS, Larson S, Nguyen J, Boudreau J, Bulin J, Rolf M. The role of enfortumab vedotin and sacituzumab govitecan in treatment of advanced bladder cancer. Am J Health-Syst Pharm. 2022;79:629–35.
Google Scholar
Fu R, Wang C, Yin T, Zhang X, Xu Y, Shi Y, et al. A novel and promising therapeutic approach for treating pancreatic cancer: Nectin‑4‑targeted antibody‑drug conjugates alone or combined with autophagy inhibitors. Int J Mol Med. 2025;55:66.
Wang Y, Nan Y, Ma C, Lu X, Wang Q, Huang X, et al. A potential strategy for bladder cancer treatment: inhibiting autophagy to enhance antitumor effects of Nectin-4-MMAE. Cell Death Dis. 2024;15:293.
Google Scholar
Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7:20–37.
Google Scholar
Cardillo TM, Sharkey RM, Rossi DL, Arrojo R, Mostafa AA, Goldenberg DM. Synthetic lethality exploitation by an anti-Trop-2-SN-38 antibody-drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2-wild-type Triple-Negative Breast Cancer. Clin Cancer Res. 2017;23:3405–15.
Google Scholar
Li Y, Li L, Fu H, Yao Q, Wang L, Lou L. Combined inhibition of PARP and ATR synergistically potentiates the antitumor activity of HER2-targeting antibody-drug conjugate in HER2-positive cancers. Am J Cancer Res. 2023;13:161–75.
Google Scholar
Kovtun Y, Noordhuis P, Whiteman KR, Watkins K, Jones GE, Harvey L, et al. IMGN779, a novel CD33-targeting antibody-drug conjugate with DNA-alkylating activity, exhibits potent antitumor activity in models of AML. Mol Cancer Ther. 2018;17:1271–9.
Google Scholar
Ghelli Luserna di Rorà A, Jandoubi M, Padella A, Ferrari A, Marranci A, Mazzotti C, et al. Exploring the role of PARP1 inhibition in enhancing antibody-drug conjugate therapy for acute leukemias: insights from DNA damage response pathway interactions. J Transl Med. 2024;22:1062.
Wei Q, Li P, Yang T, Zhu J, Sun L, Zhang Z, et al. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J Hematol Oncol. 2024;17:1.
Google Scholar
Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209.
Google Scholar
Trail PA, Dubowchik GM, Lowinger TB. Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design. Pharmacol Ther. 2018;181:126–42.
Google Scholar
Ponte JF, Ab O, Lanieri L, Lee J, Coccia J, Bartle LM, et al. Mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, potentiates the activity of standard of care therapeutics in ovarian cancer models. Neoplasia. 2016;18:775–84.
Google Scholar
Fenton MA, Tarantino P, Graff SL. Sequencing antibody drug conjugates in breast cancer: exploring future roles. Curr Oncol. 2023;30:10211–23.
Google Scholar
Rommelfanger DM, Wongthida P, Diaz RM, Kaluza KM, Thompson JM, Kottke TJ, et al. Systemic combination virotherapy for melanoma with tumor antigen-expressing vesicular stomatitis virus and adoptive T-cell transfer. Cancer Res. 2012;72:4753–64.
Google Scholar
Gonçalves MP, Farah R, Bikorimana JP, Abusarah J, El-Hachem N, Saad W, et al. A1-reprogrammed mesenchymal stromal cells prime potent antitumoral responses. iScience. 2024;27:109248.
Google Scholar
Taha Z, Crupi MJF, Alluqmani N, MacKenzie D, Vallati S, Whelan JT, et al. Complementary dual-virus strategy drives synthetic target and cognate T-cell engager expression for endogenous-antigen agnostic immunotherapy. Nat Commun. 2024;15:7267.
Google Scholar
Palma M. Advancing Breast Cancer Treatment: The Role of Immunotherapy and Cancer Vaccines in Overcoming Therapeutic Challenges. Vaccines (Basel). 2025;13:344.
Li L, Zhang D, Liu B, Lv D, Zhai J, Guan X, et al. Antibody-drug conjugates in HER2-positive breast cancer. Chin Med J (Engl). 2021;135:261–7.
Google Scholar
Jiang Y, Dong S, Wang Y. Antibody-Drug Conjugates Targeting CD30 in T-Cell Lymphomas: Clinical Progression and Mechanism. Cancers (Basel). 2025;17:496.
Song L, Qi J, Wang Z, Li X, Xiao S, Zhu H, et al. BL-M11D1, a novel CD33 antibody-drug conjugate (ADC), in patients with relapsed/ refractory acute myeloid leukemia: initial results from first-in-human phase 1 study. Blood. 2024;144:4260.
Okajima D, Yasuda S, Maejima T, Karibe T, Sakurai K, Aida T, et al. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021;20:2329–40.
Google Scholar
Journeaux T, Bernardes GJL. Homogeneous multi-payload antibody-drug conjugates. Nat Chem. 2024;16:854–70.
Google Scholar
Pan Y, Yuan C, Zeng C, Sun C, Xia L, Wang G, et al. Cancer stem cells and niches: challenges in immunotherapy resistance. Mol Cancer. 2025;24:52.
Google Scholar
Liu C, Zhang G, Xiang K, Kim Y, Lavoie RR, Lucien F, et al. Targeting the immune checkpoint B7–H3 for next-generation cancer immunotherapy. Cancer Immunol Immunother. 2022;71:1549–67.
Google Scholar
Wang X, Zhao L, Gao F, Meng Y, Yang J, Zhu M, et al. HER3-targeting Antibody-drug Conjugates Therapy for Solid Tumors: Recent Advances and Future Potentials. Curr Med Chem. 2025. https://doi.org/10.2174/0109298673358929250213093803
Choi M, Bae S, Lee D, Choi H. Abstract 2503: analytic platform for optimal target pair selection in bispecific antibody-drug conjugates using scRNA-seq and spatial transcriptomics data. Cancer Res. 2025;85:2503–2503.
Zhu Y, Ouyang Z, Du H, Wang M, Wang J, Sun H, et al. New opportunities and challenges of natural products research: when target identification meets single-cell multiomics. Acta Pharm Sin B. 2022;12:4011–39.
Google Scholar
Gray ME, Zielinski KM, Xu F, Elder KK, McKay SJ, Ojo VT, et al. A comparison of the activity, lysosomal stability, and efficacy of legumain-cleavable and cathepsin-cleavable ADC linkers. Xenobiotica. 2024;54:458–68.
Google Scholar
Sheyi R, de la Torre BG, Albericio F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics. 2022;14:396.
Watanabe T, Arashida N, Fujii T, Shikida N, Ito K, Shimbo K, et al. Exo-cleavable linkers: enhanced stability and therapeutic efficacy in antibody-drug conjugates. J Med Chem. 2024;67:18124–38.
Google Scholar
Majumder U, Zhu X, Custar D, Li D, Fang H, McGonigle S, et al. A Novel Concept for Cleavable Linkers Applicable to Conjugation Chemistry – Design. Synthesis and Characterization Chembiochem. 2025;26:e202400826.
Google Scholar
Li M, Zhao X, Yu C, Wang L. Antibody-drug conjugate overview: a state-of-the-art manufacturing process and control strategy. Pharm Res. 2024;41:419–40.
Google Scholar
Zhang J, Yang Z, Liu Y, Liu Y, Qu J, Pan X. Recent advances in smart linkage strategies for developing drug conjugates for targeted delivery. Top Curr Chem. 2025;383:13.
Google Scholar
Mountzios G, Saw SPL, Hendriks L, Menis J, Cascone T, Arrieta O, et al. Antibody-drug conjugates in NSCLC with actionable genomic alterations: optimizing smart delivery of chemotherapy to the target. Cancer Treat Rev. 2025;134:102902.
Google Scholar
Fujii T, Matsuda Y, Seki T, Shikida N, Iwai Y, Ooba Y, et al. AJICAP Second Generation: Improved Chemical Site-Specific Conjugation Technology for Antibody-Drug Conjugate Production. Bioconjug Chem. 2023;34:728–38.
Google Scholar
Matsuda Y, Seki T, Yamada K, Ooba Y, Takahashi K, Fujii T, et al. Chemical site-specific conjugation platform to improve the pharmacokinetics and therapeutic index of antibody-drug conjugates. Mol Pharm. 2021;18:4058–66.
Google Scholar
Hingorani DV. An overview of site-specific methods for achieving antibody drug conjugates with homogenous drug to antibody ratio. Expert Opin Biol Ther. 2024;24:31–6.
Google Scholar
Long J, Shao T, Wang Y, Chen T, Chen Y, Chen YL, et al. Pegylation of dipeptide linker improves therapeutic index and pharmacokinetics of antibody-drug conjugates. Bioconjug Chem. 2025;36:179–89.
Google Scholar
Zhang Y, Wang L, Cao X, Song R, Yin S, Cheng Z, et al. Evaluation of double self-immolative linker-based antibody-drug conjugate FDA022-BB05 with enhanced therapeutic potential. J Med Chem. 2024;67:19852–73.
Google Scholar
Taylor RP, Lindorfer MA. Antibody-drug conjugate adverse effects can be understood and addressed based on immune complex clearance mechanisms. Blood. 2024;144:137–44.
Google Scholar
Collins DM, Bossenmaier B, Kollmorgen G, Niederfellner G. Acquired Resistance to Antibody-Drug Conjugates. Cancers (Basel). 2019;11:394.
Loganzo F, Sung M, Gerber HP. Mechanisms of resistance to antibody-drug conjugates. Mol Cancer Ther. 2016;15:2825–34.
Google Scholar
Theile D, Wizgall P. Acquired ABC-transporter overexpression in cancer cells: transcriptional induction or Darwinian selection? Naunyn Schmiedebergs Arch Pharmacol. 2021;394:1621–32.
Google Scholar
Ps S. Zhou Y, Stelter I, Hanlon T, Bekele RT, Bellmunt J, Szallasi Z, Mouw KW: Impact of DNA repair deficiency on sensitivity to antibody-drug conjugate (ADC) payloads in bladder cancer. Bladder Cancer. 2025;11:23523735251317864.
Meyer MJ, Jenkins D, Batt D, Mosher R, Isaacs R, Hu T, et al. Abstract 1680: in vitro and in vivo activity of a highly potent and novel FGFR2/FGFR4 dual targeting antibody-drug conjugate. Cancer Res. 2015;75:1680–1680.
Xie X, Lee J, Iwase T, Kai M, Ueno NT. Emerging drug targets for triple-negative breast cancer: a guided tour of the preclinical landscape. Expert Opin Ther Targets. 2022;26:405–25.
Google Scholar
Xie X, Lee J, Fuson JA, Liu H, Iwase T, Yun K, et al. Identification of Kinase Targets for Enhancing the Antitumor Activity of Eribulin in Triple-Negative Breast Cell Lines. Biomedicines. 2023;11:735.
Shi Y, Bashian EE, Hou Y, Wu P. Chemical immunology: Recent advances in tool development and applications. Cell Chem Biol. 2024.
Liu B, Zhou H, Tan L, Siu KTH, Guan XY. Exploring treatment options in cancer: tumor treatment strategies. Signal Transduct Target Ther. 2024;9:175.
Google Scholar
Patel AM, Willingham A, Cheng AC, Tomazela D, Bowman E, Kofman E, et al. Design and optimization of selectivity-tunable Toll-like receptor 7/8 agonists as novel antibody-drug conjugate payloads. J Med Chem. 2024;67:15756–79.
Google Scholar
Ocker M, Mayr C, Huber-Cantonati P, Kiesslich T, Neureiter D. New frontiers in the pharmacological management of biliary tract carcinomas: the emerging role of drug conjugates. Expert Opin Pharmacother. 2025;26:887–96.
Google Scholar
Zhu H, Wang J, Zhang Q, Pan X, Zhang J. Novel strategies and promising opportunities for targeted protein degradation: an innovative therapeutic approach to overcome cancer resistance. Pharmacol Ther. 2023;244:108371.
Google Scholar
Yu P, Zhu C, You X, Gu W, Wang X, Wang Y, et al. The combination of immune checkpoint inhibitors and antibody-drug conjugates in the treatment of urogenital tumors: a review insights from phase 2 and 3 studies. Cell Death Dis. 2024;15:433.
Google Scholar
Peters S, Loi S, André F, Chandarlapaty S, Felip E, Finn SP, et al. Antibody-drug conjugates in lung and breast cancer: current evidence and future directions-a position statement from the ETOP IBCSG Partners Foundation. Ann Oncol. 2024;35:607–29.
Google Scholar
Davis AA, Hesse J, Pereira PMR, Ma CX. Novel treatment approaches utilizing antibody-drug conjugates in breast cancer. NPJ Breast Cancer. 2025;11:42.
Google Scholar
Afzal MZ, Vahdat LT. Evolving Management of Breast Cancer in the Era of Predictive Biomarkers and Precision Medicine. J Pers Med. 2024;14:719.
Garg P, Malhotra J, Kulkarni P, Horne D, Salgia R, Singhal SS. Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells. Cancers (Basel). 2024;16:2478.
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50.
Google Scholar
Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80.
Google Scholar
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88.
Google Scholar
Chang HL, Schwettmann B, McArthur HL, Chan IS. Antibody-drug conjugates in breast cancer: overcoming resistance and boosting immune response. J Clin Invest. 2023;133:e172156.
Wittwer NL, Staudacher AH, Liapis V, Cardarelli P, Warren H, Brown MP. An anti-mesothelin targeting antibody drug conjugate induces pyroptosis and ignites antitumor immunity in mouse models of cancer. J Immunother Cancer. 2023;11:e006274.
Valsasina B, Orsini P, Caruso M, Albanese C, Ciavolella A, Cucchi U, et al. Novel Thienoduocarmycin-Trastuzumab ADC demonstrates strong antitumor efficacy with favorable safety profile in preclinical studies. Mol Cancer Ther. 2023;22:1465–78.
Google Scholar
Matsumura Y. Cancer stromal targeting therapy to overcome the pitfall of EPR effect. Adv Drug Deliv Rev. 2020;154–155:142–50.
Google Scholar
Al Sbihi A, Alasfour M, Pongas G. Innovations in Antibody-Drug Conjugate (ADC) in the Treatment of Lymphoma. Cancers (Basel). 2024;16:827.
Abuhelwa Z, Alloghbi A, Nagasaka M. A comprehensive review on antibody-drug conjugates (ADCs) in the treatment landscape of non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2022;106:102393.
Google Scholar
Scheuher B, Ghusinga KR, McGirr K, Nowak M, Panday S, Apgar J, et al. Towards a platform quantitative systems pharmacology (QSP) model for preclinical to clinical translation of antibody drug conjugates (ADCs). J Pharmacokinet Pharmacodyn. 2024;51:429–47.
Google Scholar
Drake PM, Rabuka D. Recent developments in ADC technology: preclinical studies signal future clinical trends. BioDrugs. 2017;31:521–31.
Google Scholar
Lucas AT, Price LSL, Schorzman AN, Storrie M, Piscitelli JA, Razo J, et al. Factors Affecting the Pharmacology of Antibody-Drug Conjugates. Antibodies (Basel). 2018;7:10.
Williams M, Spreafico A, Vashisht K, Hinrichs MJ. Patient selection strategies to maximize therapeutic index of antibody-drug conjugates: prior approaches and future directions. Mol Cancer Ther. 2020;19:1770–83.
Google Scholar
Asiimwe IG, Chtiba N, Mouksassi S, Pillai GC, Peter RM, Yuen E, et al. Postmarketing Assessment of Antibody-Drug Conjugates: Proof-of-Concept Using Model-Based Meta-Analysis and a Clinical Utility Index Approach. CPT Pharmacometrics Syst Pharmacol. 2025.
Leleu X, Bobin A, Herbelin A, Gombert JM, Rajkumar SV. Time for a paradigm shift in immunotherapy-based BCMA/CD3 bispecific drug development in multiple myeloma. Leukemia. 2025;39:1593-1594.
Wang Y, Gao X, Dan M, Hui X, Yao B, Zhang Y, et al. Abstract 4338: SYS6042, a novel anti-TROP2 pH-sensitive antibody-drug conjugate (ADC) with improved therapeutic window. Cancer Res. 2025;85:4338–4338.
Tarantino P, Ricciuti B, Pradhan SM, Tolaney SM. Optimizing the safety of antibody-drug conjugates for patients with solid tumours. Nat Rev Clin Oncol. 2023;20:558–76.
Google Scholar
Gong J, Zhang W, Balthasar JP. Camptothein-Based Anti-Cancer Therapies and Strategies to Improve Their Therapeutic Index. Cancers (Basel). 2025;17:1032.
Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117:1736–42.
Google Scholar
Giugliano F, Corti C, Tarantino P, Michelini F, Curigliano G. Bystander effect of antibody-drug conjugates: fact or fiction? Curr Oncol Rep. 2022;24:809–17.
Google Scholar
Ran R, Chen X, Yang J, Xu B. Immunotherapy in breast cancer: current landscape and emerging trends. Exp Hematol Oncol. 2025;14:77.
Google Scholar
Stumpo S, Carlini A, Mantuano F, Di Federico A, Melotti B, Sperandi F, et al. Efficacy and Safety of TROP-2-Targeting Antibody-Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data. Cancers (Basel). 2025;17:1750.
McCombs JR, Owen SC. Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J. 2015;17:339–51.
Google Scholar
Tang H, Liu Y, Yu Z, Sun M, Lin L, Liu W, et al. The analysis of key factors related to ADCs structural design. Front Pharmacol. 2019;10:373.
Google Scholar
Masters JC, Nickens DJ, Xuan D, Shazer RL, Amantea M. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Invest New Drugs. 2018;36:121–35.
Google Scholar
Bhakta S, Raab H, Junutula JR. Engineering THIOMABs for site-specific conjugation of thiol-reactive linkers. Methods Mol Biol. 2013;1045:189–203.
Google Scholar
Zacharias N, Podust VN, Kajihara KK, Leipold D, Del Rosario G, Thayer D, et al. A homogeneous high-DAR antibody-drug conjugate platform combining THIOMAB antibodies and XTEN polypeptides. Chem Sci. 2022;13:3147–60.
Google Scholar
Dennler P, Chiotellis A, Fischer E, Brégeon D, Belmant C, Gauthier L, et al. Transglutaminase-based chemo-enzymatic conjugation approach yields homogeneous antibody-drug conjugates. Bioconjug Chem. 2014;25:569–78.
Google Scholar
Kopp A, Hofsess S, Cardillo TM, Govindan SV, Donnell J, Thurber GM. Antibody-drug conjugate Sacituzumab Govitecan drives efficient tissue penetration and rapid intracellular drug release. Mol Cancer Ther. 2023;22:102–11.
Google Scholar
Mathi GR, Lee BS, Chun Y, Shin S, Kweon S, Go A, et al. Design, synthesis and biological evaluation of camptothecin analogue FL118 as a payload for antibody-drug conjugates in targeted cancer therapy. Bioorg Med Chem Lett. 2025;118:130085.
Google Scholar
Catenacci DV. Next-generation clinical trials: novel strategies to address the challenge of tumor molecular heterogeneity. Mol Oncol. 2015;9:967–96.
Google Scholar
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66.
Google Scholar
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
Google Scholar
Saini KS, Punie K, Twelves C, Bortini S, de Azambuja E, Anderson S, et al. Antibody-drug conjugates, immune-checkpoint inhibitors, and their combination in breast cancer therapeutics. Expert Opin Biol Ther. 2021;21:945–62.
Google Scholar
Gilad Y, Gellerman G, Lonard DM, O’Malley BW. Drug Combination in Cancer Treatment-From Cocktails to Conjugated Combinations. Cancers (Basel). 2021;13:669.
Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25–39.
Google Scholar
Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71.
Google Scholar
Milbury CA, Creeden J, Yip WK, Smith DL, Pattani V, Maxwell K, et al. Clinical and analytical validation of FoundationOne®CDx, a comprehensive genomic profiling assay for solid tumors. PLoS ONE. 2022;17:e0264138.
Google Scholar
Robbins CJ, Bates KM, Rimm DL. HER2 testing: evolution and update for a companion diagnostic assay. Nat Rev Clin Oncol. 2025;22:408–23.
Google Scholar
Goldberg KB, Blumenthal GM, McKee AE, Pazdur R. The FDA Oncology Center of Excellence and precision medicine. Exp Biol Med (Maywood). 2018;243:308–12.
Google Scholar
Coats S, Williams M, Kebble B, Dixit R, Tseng L, Yao NS, et al. Antibody-drug conjugates: future directions in clinical and translational strategies to improve the therapeutic index. Clin Cancer Res. 2019;25:5441–8.
Google Scholar
Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–50.
Google Scholar
Penault-Llorca F, Viale G. Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann Oncol. 2012;23(Suppl 6):vi19-22.
Google Scholar
Bartelink IH, Jones EF, Shahidi-Latham SK, Lee PRE, Zheng Y, Vicini P, et al. Tumor drug penetration measurements could be the neglected piece of the personalized cancer treatment puzzle. Clin Pharmacol Ther. 2019;106:148–63.
Google Scholar
Parakh S, Nicolazzo J, Scott AM, Gan HK. Antibody drug conjugates in glioblastoma – is there a future for them? Front Oncol. 2021;11:718590.
Google Scholar
Guzzeloni V, Veschini L, Pedica F, Ferrero E, Ferrarini M. 3D Models as a Tool to Assess the Anti-Tumor Efficacy of Therapeutic Antibodies: Advantages and Limitations. Antibodies (Basel). 2022;11:46.
Gan HK, Parakh S, Osellame LD, Cher L, Uccellini A, Hafeez U, et al. Antibody drug conjugates for glioblastoma: current progress towards clinical use. Expert Opin Biol Ther. 2023;23:1089–102.
Google Scholar
Yoon J, Oh DY. HER2-targeted therapies beyond breast cancer – an update. Nat Rev Clin Oncol. 2024;21:675–700.
Google Scholar
Tarantino P, Gupta H, Hughes ME, Files J, Strauss S, Kirkner G, et al. Comprehensive genomic characterization of HER2-low and HER2-0 breast cancer. Nat Commun. 2023;14:7496.
Google Scholar
Lyski RD, Bou LB, Lau UY, Meyer DW, Cochran JH, Okeley NM, et al. Development of novel antibody-camptothecin conjugates. Mol Cancer Ther. 2021;20:329–39.
Google Scholar
Belluomini L, Sposito M, Avancini A, Insolda J, Milella M, Rossi A, et al. Unlocking New Horizons in Small-Cell Lung Cancer Treatment: The Onset of Antibody-Drug Conjugates. Cancers (Basel). 2023;15:5368.
Van Dongen GA, Huisman MC, Boellaard R, Harry Hendrikse N, Windhorst AD, Visser GW, et al. 89Zr-immuno-PET for imaging of long circulating drugs and disease targets: why, how and when to be applied? Q J Nucl Med Mol Imaging. 2015;59:18–38.
Google Scholar
Kristensen LK, Christensen C, Jensen MM, Agnew BJ, Schjöth-Frydendahl C, Kjaer A, et al. Site-specifically labeled (89)Zr-DFO-trastuzumab improves immuno-reactivity and tumor uptake for immuno-PET in a subcutaneous HER2-positive xenograft mouse model. Theranostics. 2019;9:4409–20.
Google Scholar
Ma D, Dai LJ, Wu XR, Liu CL, Zhao S, Zhang H, Chen L, Xiao Y, Li M, Zhao YZ, et al: Spatial determinants of antibody-drug conjugate SHRA1811 efficacy in neoadjuvant treatment for HER2-positive breast cancer. Cancer Cell 2025, 43:1061–1075.e1067.
Massa D, Tosi A, Rosato A, Guarneri V, Dieci MV. Multiplexed In Situ Spatial Protein Profiling in the Pursuit of Precision Immuno-Oncology for Patients with Breast Cancer. Cancers (Basel). 2022;14:4885.
Maecker H, Jonnalagadda V, Bhakta S, Jammalamadaka V, Junutula JR. Exploration of the antibody-drug conjugate clinical landscape. MAbs. 2023;15:2229101.
Google Scholar
Pommier Y, Thomas A. New life of Topoisomerase I inhibitors as antibody-drug conjugate warheads. Clin Cancer Res. 2023;29:991–3.
Google Scholar
Bhushan A, Misra P. Economics of antibody drug conjugates (ADCs): innovation, investment and market dynamics. Curr Oncol Rep. 2024;26:1224–35.
Google Scholar
Crescioli S, Kaplon H, Wang L, Visweswaraiah J, Kapoor V, Reichert JM. Antibodies to watch in 2025. MAbs. 2025;17:2443538.
Google Scholar
Kiss B, Borbély J. Business Risk Mitigation in the Development Process of New Monoclonal Antibody Drug Conjugates for Cancer Treatment. Pharmaceutics. 2023;15:1761.
Beck A, Dumontet C, Joubert N. Antibody-drug conjugates in oncology. New strategies in development. Med Sci (Paris). 2019;35:1043-1053.
Yang Q, Liu Y. Technical, preclinical, and clinical developments of Fc-glycan-specific antibody-drug conjugates. RSC Med Chem. 2025;16:50–62.
Google Scholar
Frigerio M, Kyle AF. The chemical design and synthesis of linkers used in antibody drug conjugates. Curr Top Med Chem. 2017;17:3393–424.
Google Scholar
de Bever L, Popal S, van Schaik J, Rubahamya B, van Delft FL, Thurber GM, et al. Generation of DAR1 antibody-drug conjugates for ultrapotent payloads using tailored GlycoConnect technology. Bioconjug Chem. 2023;34:538–48.
Google Scholar
Kondrashov A, Sapkota S, Sharma A, Riano I, Kurzrock R, Adashek JJ. Antibody-Drug Conjugates in Solid Tumor Oncology: An Effectiveness Payday with a Targeted Payload. Pharmaceutics. 2023;15:2160.
Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2019;380:741–51.
Google Scholar
Bai JPF, Musante CJ, Petanceska S, Zhang L, Zhao L, Zhao P. American Society for Clinical Pharmacology and Therapeutics 2019 annual meeting pre-conferences. CPT Pharmacometrics Syst Pharmacol. 2019;8:333–5.
Google Scholar
American Society for Clinical Pharmacology and Therapeutics. Clin Pharmacol Ther. 2022;111(Suppl 1):S5-s80.
Zimmerman BS, Esteva FJ. Next-Generation HER2-Targeted Antibody-Drug Conjugates in Breast Cancer. Cancers (Basel). 2024;16:800.
Wei R, Zhang W, Yang F, Li Q, Wang Q, Liu N, et al. Dual targeting non-overlapping epitopes in HER2 domain IV substantially enhanced HER2/HER2 homodimers and HER2/EGFR heterodimers internalization leading to potent antitumor activity in HER2-positive human gastric cancer. J Transl Med. 2024;22:641.
Google Scholar
Chan K, Sathyamurthi PS, Queisser MA, Mullin M, Shrives H, Coe DM, et al. Antibody-proteolysis targeting chimera conjugate enables selective degradation of receptor-interacting serine/threonine-protein kinase 2 in HER2+ cell lines. Bioconjug Chem. 2023;34:2049–54.
Google Scholar
Mckertish CM, Kayser V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines. 2021;9:872.
Krytska K, Casey CE, Pogoriler J, Martinez D, Rathi KS, Farrel A, et al. Evaluation of the DLL3-targeting antibody-drug conjugate rovalpituzumab tesirine in preclinical models of neuroblastoma. Cancer Res Commun. 2022;2:616–23.
Google Scholar
Fabrizio FP, Muscarella LA, Rossi A. B7–H3/CD276 and small-cell lung cancer: what’s new? Transl Oncol. 2024;39:101801.
Google Scholar
Mair MJ, Bartsch R, Le Rhun E, Berghoff AS, Brastianos PK, Cortes J, et al. Understanding the activity of antibody-drug conjugates in primary and secondary brain tumours. Nat Rev Clin Oncol. 2023;20:372–89.
Google Scholar
Zeng H, Ning W, Liu X, Luo W, Xia N. Unlocking the potential of bispecific ADCs for targeted cancer therapy. Front Med. 2024;18:597–621.
Google Scholar
Hong Y, Nam SM, Moon A. Antibody-drug conjugates and bispecific antibodies targeting cancers: applications of click chemistry. Arch Pharm Res. 2023;46:131–48.
Google Scholar
Schlam I, Moges R, Morganti S, Tolaney SM, Tarantino P. Next-generation antibody-drug conjugates for breast cancer: moving beyond HER2 and TROP2. Crit Rev Oncol Hematol. 2023;190:104090.
Google Scholar
Yajaman DR, Oh Y, Trevino JG, Harrell JC. Advancing Antibody-Drug Conjugates: Precision Oncology Approaches for Breast and Pancreatic Cancers. Cancers (Basel). 2025;17:1792.
Diamantis N, Banerji U. Antibody-drug conjugates–an emerging class of cancer treatment. Br J Cancer. 2016;114:362–7.
Google Scholar
Leyton JV. The endosomal-lysosomal system in ADC design and cancer therapy. Expert Opin Biol Ther. 2023;23:1067–76.
Google Scholar
Zhang K, Yang X, Wang Y, Yu Y, Huang N, Li G, et al. Artificial intelligence in drug development. Nat Med. 2025;31:45–59.
Google Scholar
Sobhani N, D’Angelo A, Pittacolo M, Mondani G, Generali D. Future AI Will Most Likely Predict Antibody-Drug Conjugate Response in Oncology: A Review and Expert Opinion. Cancers (Basel). 2024;16:3089.
Sapra P, Hooper AT, O’Donnell CJ, Gerber HP. Investigational antibody drug conjugates for solid tumors. Expert Opin Investig Drugs. 2011;20:1131–49.
Google Scholar
Haddish-Berhane N, Shah DK, Ma D, Leal M, Gerber HP, Sapra P, et al. On translation of antibody drug conjugates efficacy from mouse experimental tumors to the clinic: a PK/PD approach. J Pharmacokinet Pharmacodyn. 2013;40:557–71.
Google Scholar
Zhang DE, He T, Shi T, Huang K, Peng A. Trends in the research and development of peptide drug conjugates: artificial intelligence aided design. Front Pharmacol. 2025;16:1553853.
Google Scholar
Angiolini L, Manetti F, Spiga O, Tafi A, Visibelli A, Petricci E. Machine learning for predicting the drug-to-antibody ratio (DAR) in the synthesis of antibody-drug conjugates (ADCs). J Chem Inf Model. 2025;65:5847–55.
Google Scholar
Sexton CE, Bitan G, Bowles KR, Brys M, Buée L, Maina MB, et al. Novel avenues of tau research. Alzheimers Dement. 2024;20:2240–61.
Google Scholar
Liu J, He M. Construction and validation of a novel immunological model to predict prognosis in pancreatic ductal adenocarcinoma. Int Immunopharmacol. 2024;134:112266.
Google Scholar
Janelle V, Rulleau C, Del Testa S, Carli C, Delisle JS. T-cell immunotherapies targeting histocompatibility and tumor antigens in hematological malignancies. Front Immunol. 2020;11:276.
Google Scholar
Xu Y, Su GH, Ma D, Xiao Y, Shao ZM, Jiang YZ. Technological advances in cancer immunity: from immunogenomics to single-cell analysis and artificial intelligence. Signal Transduct Target Ther. 2021;6:312.
Google Scholar
Srinivasan B. Time-evolved metrics for safety pharmacological assessments of small molecules and biologics. Br J Pharmacol. 2025;182(13):2831–41.
Google Scholar
Zhang Y, Luo M, Wu P, Wu S, Lee TY, Bai C. Application of Computational Biology and Artificial Intelligence in Drug Design. Int J Mol Sci. 2022;23:13568.
Tedeschini T, Campara B, Grigoletto A, Zanotto I, Cannella L, Gabbia D, et al. Optimization of a pendant-shaped PEGylated linker for antibody-drug conjugates. J Control Release. 2024;375:74–89.
Google Scholar
Li JH, Liu L, Zhao XH. Precision targeting in oncology: the future of conjugated drugs. Biomed Pharmacother. 2024;177:117106.
Google Scholar
Jeon JH, Woo Kim S, Kim YJ, Park JW, Eun Moon J, Beom Lee Y, et al. Synthesis and evaluation of antibody-drug conjugates with high drug-to-antibody ratio using dimaleimide-DM1 as a linker- payload. Bioorg Chem. 2024;149:107504.
Google Scholar
Wang C, Xu P, Zhang L, Huang J, Zhu K, Luo C. Current strategies and applications for precision drug design. Front Pharmacol. 2018;9:787.
Google Scholar
Tong JTW, Harris PWR, Brimble MA, Kavianinia I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules. 2021;26:5847.
Li M, Mei S, Yang Y, Shen Y, Chen L. Strategies to mitigate the on- and off-target toxicities of recombinant immunotoxins: an antibody engineering perspective. Antib Ther. 2022;5:164–76.
Google Scholar
Rao M, McDuffie E, Sachs C. Artificial Intelligence/Machine Learning-Driven Small Molecule Repurposing via Off-Target Prediction and Transcriptomics. Toxics. 2023;11:875.
Guo Y, Shen Z, Zhao W, Lu J, Song Y, Shen L, et al. Rational identification of novel antibody-drug conjugate with high bystander killing effect against heterogeneous tumors. Adv Sci (Weinh). 2024;11:e2306309.
Google Scholar
Dewaker V, Morya VK, Kim YH, Park ST, Kim HS, Koh YH. Revolutionizing oncology: the role of artificial intelligence (AI) as an antibody design, and optimization tools. Biomark Res. 2025;13:52.
Google Scholar
Chen L, Li B, Chen Y, Lin M, Zhang S, Li C, Pang Y, Wang L: ADCNet: a unified framework for predicting the activity of antibody-drug conjugates. Brief Bioinform 2025; 26:bbaf228
Ghemrawi R, Abuamer L, Kremesh S, Hussien G, Ahmed R, Mousa W, et al. Revolutionizing Cancer Treatment: Recent Advances in Immunotherapy. Biomedicines. 2024;12:2158.
Huemer F, Leisch M, Geisberger R, Melchardt T, Rinnerthaler G, Zaborsky N, et al. Combination Strategies for Immune-Checkpoint Blockade and Response Prediction by Artificial Intelligence. Int J Mol Sci. 2020;21:2856.
Larose ÉA, Hua X, Yu S, Pillai AT, Yi Z, Yu H. Antibody-drug conjugates in breast cancer treatment: resistance mechanisms and the role of therapeutic sequencing. Cancer Drug Resist. 2025;8:11.
Google Scholar
Mitra A, Kumar A, Amdare NP, Pathak R. Current Landscape of Cancer Immunotherapy: Harnessing the Immune Arsenal to Overcome Immune Evasion. Biology (Basel). 2024;13:307.
Tang Y, Tang F, Yang Y, Zhao L, Zhou H, Dong J, et al. Real-time analysis on drug-antibody ratio of antibody-drug conjugates for synthesis, process optimization, and quality control. Sci Rep. 2017;7:7763.
Google Scholar
Harkos C, Hadjigeorgiou AG, Voutouri C, Kumar AS, Stylianopoulos T, Jain RK. Using mathematical modelling and AI to improve delivery and efficacy of therapies in cancer. Nat Rev Cancer. 2025;25:324–40.
Google Scholar
Wu S, Yue M, Zhang J, Li X, Li Z, Zhang H, et al. The role of artificial intelligence in accurate interpretation of HER2 immunohistochemical scores 0 and 1+ in breast cancer. Mod Pathol. 2023;36:100054.
Google Scholar
Tao Y, Lu Y, Yu B, Wang Y. Molecular glue meets antibody: next-generation antibody-drug conjugates. Trends Pharmacol Sci. 2025;46:520–34.
Google Scholar
Pacholczak-Madej R, Meluch M, Püsküllüoğlu M. Sequencing of antibody drug conjugates in breast cancer: evidence gap and future directions. Biochim Biophys Acta Rev Cancer. 2025;1880:189369.
Google Scholar
Liu K, Ai Y, Tan HY, Yuan J, Meissen JK, Zhang Y, et al. Streamlined high-throughput data analysis workflow for antibody-drug conjugate biotransformation characterization. Anal Chem. 2025;97:5919–25.
Google Scholar
Jin Y, Schladetsch MA, Huang X, Balunas MJ, Wiemer AJ. Stepping forward in antibody-drug conjugate development. Pharmacol Ther. 2022;229:107917.
Google Scholar
Li J, Wang Q, Xia G, Adilijiang N, Li Y, Hou Z, et al. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics. 2023;15:2233.
Ertas YN, Abedi Dorcheh K, Akbari A, Jabbari E. Nanoparticles for Targeted Drug Delivery to Cancer Stem Cells: A Review of Recent Advances. Nanomaterials (Basel). 2021;11:1755.
Duan H, Liu Y, Gao Z, Huang W. Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm Sin B. 2021;11:55–70.
Google Scholar
Gao Q, Feng J, Liu W, Wen C, Wu Y, Liao Q, et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv Drug Deliv Rev. 2022;188:114445.
Google Scholar
Anand U, Dey A, Chandel AKS, Sanyal R, Mishra A, Pandey DK, et al. Cancer chemotherapy and beyond: current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023;10:1367–401.
Google Scholar
Chidambaram M, Manavalan R, Kathiresan K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J Pharm Pharm Sci. 2011;14:67–77.
Google Scholar
Juan A, Cimas FJ, Bravo I, Pandiella A, Ocaña A, Alonso-Moreno C. Antibody Conjugation of Nanoparticles as Therapeutics for Breast Cancer Treatment. Int J Mol Sci. 2020;21:6018.
Hamdy NM, Eskander G, Basalious EB. Insights on the dynamic innovative tumor targeted-nanoparticles-based drug delivery systems activation techniques. Int J Nanomedicine. 2022;17:6131–55.
Google Scholar
Kumbhar PR, Kumar P, Lasure A, Velayutham R, Mandal D. An updated landscape on nanotechnology-based drug delivery, immunotherapy, vaccinations, imaging, and biomarker detections for cancers: recent trends and future directions with clinical success. Discover Nano. 2023;18:156.
Google Scholar
Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–57.
Google Scholar
Liu R, Luo C, Pang Z, Zhang J, Ruan S, Wu M, et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin Chem Lett. 2023;34:107518.
Google Scholar
Gao C, Bhattarai P, Chen M, Zhang N, Hameed S, Yue X, et al. Amphiphilic drug conjugates as nanomedicines for combined cancer therapy. Bioconjug Chem. 2018;29:3967–81.
Google Scholar
Cruz E, Kayser V. Synthesis and Enhanced Cellular Uptake In Vitro of Anti-HER2 Multifunctional Gold Nanoparticles. Cancers (Basel). 2019;11:870.
Juan A, Cimas FJ, Bravo I, Pandiella A, Ocaña A, Alonso-Moreno C. An Overview of Antibody Conjugated Polymeric Nanoparticles for Breast Cancer Therapy. Pharmaceutics. 2020;12:802.
Yan S, Na J, Liu X, Wu P. Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy. Pharmaceutics. 2024;16:248.
Spada A, Gerber-Lemaire S. Surface Functionalization of Nanocarriers with Anti-EGFR Ligands for Cancer Active Targeting. Nanomaterials (Basel). 2025;15:158.
Fu Z, Xiang J. Aptamer-Functionalized Nanoparticles in Targeted Delivery and Cancer Therapy. Int J Mol Sci. 2020;21:9123.
Kim M, Kim DM, Kim KS, Jung W, Kim DE. Applications of Cancer Cell-Specific Aptamers in Targeted Delivery of Anticancer Therapeutic Agents. Molecules. 2018;23:830.
Rizwanullah M, Ahmad MZ, Ghoneim MM, Alshehri S, Imam SS, Md S, et al. Receptor-Mediated Targeted Delivery of Surface-ModifiedNanomedicine in Breast Cancer: Recent Update and Challenges. Pharmaceutics. 2021;13:2039.
Akhtar MJ, Ahamed M, Alhadlaq HA, Alrokayan SA, Kumar S. Targeted anticancer therapy: overexpressed receptors and nanotechnology. Clin Chim Acta. 2014;436:78–92.
Google Scholar
Kumar R, Dkhar DS, Kumari R. Divya, Mahapatra S, Srivastava A, Dubey VK, Chandra P: Ligand conjugated lipid-based nanocarriers for cancer theranostics. Biotechnol Bioeng. 2022;119:3022–43.
Google Scholar
Brylev VA, Ryabukhina EV, Nazarova EV, Samoylenkova NS, Gulyak EL, Sapozhnikova KA, et al. Towards Aptamer-Targeted Drug Delivery to Brain Tumors: The Synthesis of Ramified Conjugates of an EGFR-Specific Aptamer with MMAE on a Cathepsin B-Cleavable Linker. Pharmaceutics. 2024;16:1434.
Razpotnik R, Novak N, Čurin Šerbec V, Rajcevic U. Targeting malignant brain tumors with antibodies. Front Immunol. 2017;8:1181.
Google Scholar
Agnihotri TG, Jadhav GS, Sahu B, Jain A. Recent trends of bioconjugated nanomedicines through nose-to-brain delivery for neurological disorders. Drug Deliv Transl Res. 2022;12:3104–20.
Google Scholar
Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23:858–83.
Google Scholar
Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–305.
Google Scholar
Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release. 2012;161:264–73.
Google Scholar
Tashima T. Brain Cancer Chemotherapy through a Delivery System across the Blood-Brain Barrier into the Brain Based on Receptor-Mediated Transcytosis Using Monoclonal Antibody Conjugates. Biomedicines. 2022;10:1597.
Wang L, Shi Y, Jiang J, Li C, Zhang H, Zhang X, et al. Micro-nanocarriers based drug delivery technology for blood-brain barrier crossing and brain tumor targeting therapy. Small. 2022;18:e2203678.
Google Scholar
Vieira DB, Gamarra LF. Getting into the brain: liposome-based strategies for effective drug delivery across the blood-brain barrier. Int J Nanomedicine. 2016;11:5381–414.
Google Scholar
Bashyal S, Thapa C, Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J Control Release. 2022;348:723–44.
Google Scholar
Basyoni AE, Atta A, Salem MM, Mohamed TM. Harnessing exosomes for targeted drug delivery systems to combat brain cancer. Cancer Cell Int. 2025;25:150.
Google Scholar
Khatami SH, Karami N, Taheri-Anganeh M, Taghvimi S, Tondro G, Khorsand M, et al. Exosomes: promising delivery tools for overcoming blood-brain barrier and glioblastoma therapy. Mol Neurobiol. 2023;60:4659–78.
Google Scholar
Kansız S, Elçin YM. Advanced liposome and polymersome-based drug delivery systems: considerations for physicochemical properties, targeting strategies and stimuli-sensitive approaches. Adv Colloid Interface Sci. 2023;317:102930.
Google Scholar
Chen H, Luo Q, Wang J, He H, Luo W, Zhang L, et al. Response of pH-sensitive doxorubicin nanoparticles on complex tumor microenvironments by tailoring multiple physicochemical properties. ACS Appl Mater Interfaces. 2020;12:22673–86.
Google Scholar
Li HJ, Du JZ, Liu J, Du XJ, Shen S, Zhu YH, et al. Smart superstructures with ultrahigh pH-sensitivity for targeting acidic tumor microenvironment: instantaneous size switching and improved tumor penetration. ACS Nano. 2016;10:6753–61.
Google Scholar
Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials. 2016;85:152–67.
Google Scholar
Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, et al. Ph-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32:693–710.
Google Scholar
Xie A, Hanif S, Ouyang J, Tang Z, Kong N, Kim NY, et al. Stimuli-responsive prodrug-based cancer nanomedicine. EBioMedicine. 2020;56:102821.
Google Scholar
Qian L, Lin X, Gao X, Khan RU, Liao JY, Du S, et al. The Dawn of a New Era: Targeting the “Undruggables” with Antibody-Based Therapeutics. Chem Rev. 2023;123:7782–853.
Google Scholar
Wei D, Sun Y, Zhu H, Fu Q. Stimuli-responsive polymer-based nanosystems for cancer theranostics. ACS Nano. 2023;17:23223–61.
Google Scholar
Shan X, Gong X, Li J, Wen J, Li Y, Zhang Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12:3028–48.
Google Scholar
He H, Liu L, Morin EE, Liu M, Schwendeman A. Survey of clinical translation of cancer nanomedicines-lessons learned from successes and failures. Acc Chem Res. 2019;52:2445–61.
Google Scholar
Working PK, S. NM, K. HS, E. M, J. V, and Lasic DD: Pharmacokinetics, Biodistribution and Therapeutic Efficacy of Doxorubicin Encapsulated in Stealth® Liposomes (Doxil®). Journal of Liposome Research 1994, 4:667–687.
Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34.
Google Scholar
Al-Taie A, Özcan Bülbül E. A paradigm use of monoclonal antibodies-conjugated nanoparticles in breast cancer treatment: current status and potential approaches. J Drug Target. 2024;32:45–56.
Google Scholar
Li Q, Li W, Xu K, Xing Y, Shi H, Jing Z, et al. PEG Linker Improves Antitumor Efficacy and Safety of Affibody-Based Drug Conjugates. Int J Mol Sci. 2021;22:1540.
Brandl F, Busslinger S, Zangemeister-Wittke U, Plückthun A. Optimizing the anti-tumor efficacy of protein-drug conjugates by engineering the molecular size and half-life. J Control Release. 2020;327:186–97.
Google Scholar
Adhikari A, Chen IA. Antibody-nanoparticle conjugates in therapy: combining the best of two worlds. Small. 2025;21:e2409635.
Google Scholar
Santin AD, Vergote I, González-Martín A, Moore K, Oaknin A, Romero I, et al. Safety and activity of anti-mesothelin antibody-drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian cancer: multicenter, phase Ib dose escalation and expansion study. Int J Gynecol Cancer. 2023;33:562–70.
Google Scholar
Quanz M, Hagemann UB, Zitzmann-Kolbe S, Stelte-Ludwig B, Golfier S, Elbi C, et al. Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expressing human ovarian cancer models. Oncotarget. 2018;9:34103–21.
Google Scholar
Li J, Al Faruque H, Li S, Sima M, Sborov D, Hu-Lieskovan S, et al. PD-L1 targeted antibody-polymer-epirubicin conjugate prolongs survival in a preclinical murine model of advanced ovarian cancer. J Control Release. 2025;382:113682.
Google Scholar
Torres ETR, Emens LA. Emerging combination immunotherapy strategies for breast cancer: dual immune checkpoint modulation, antibody-drug conjugates and bispecific antibodies. Breast Cancer Res Treat. 2022;191:291–302.
Google Scholar
Hao Y, Ji Z, Zhou H, Wu D, Gu Z, Wang D, et al. Lipid-based nanoparticles as drug delivery systems for cancer immunotherapy. MedComm. 2023;4:e339.
Google Scholar
Fobian SF, Cheng Z, Ten Hagen TLM. Smart Lipid-Based Nanosystems for Therapeutic Immune Induction against Cancers: Perspectives and Outlooks. Pharmaceutics. 2021;14:26.
Iyer KA, Ivanov J, Tenchov R, Ralhan K, Rodriguez Y, Sasso JM, et al. Emerging targets and therapeutics in immuno-oncology: insights from landscape analysis. J Med Chem. 2024;67:8519–44.
Google Scholar
Ejigah V, Mandala B, Akala EO. Nanotechnology in the development of small and large molecule tyrosine kinase inhibitors and immunotherapy for the treatment of HER2-positive breast cancer. J Cancer Metastasis Res. 2022;4:6–22.
Google Scholar
Lee JH, Chapman DV, Saltzman WM. Nanoparticle targeting with antibodies in the central nervous system. BME Front. 2023;4:0012.
Google Scholar
Battogtokh G, Obidiro O, Akala EO. Recent Developments in Combination Immunotherapy with Other Therapies and Nanoparticle-Based Therapy for Triple-Negative Breast Cancer (TNBC). Cancers (Basel). 2024;16:2012.
Hoimes CJ, Flaig TW, Milowsky MI, Friedlander TW, Bilen MA, Gupta S, et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol. 2023;41:22–31.
Google Scholar
Gupta S, Loriot Y, Van der Heijden MS, Bedke J, Valderrama BP, Kikuchi E, et al. Enfortumab vedotin plus pembrolizumab versus chemotherapy in patients with previously untreated locally advanced or metastatic urothelial cancer (EV-302): patient-reported outcomes from an open-label, randomised, controlled, phase 3 study. Lancet Oncol. 2025;26:795–805.
Google Scholar
Patnaik A, Call JA, Spreafico A, Nabell L, Yan M, Forero-Torres A, et al. Phase 1 study of SGN-PDL1V, a novel, investigational vedotin antibody–drug conjugate directed to PD-L1, in patients with advanced solid tumors (SGNPDL1V-001, trial in progress). J Clin Oncol. 2022;40:TPS3154–TPS3154.
Francis DM, Manspeaker MP, Archer PA, Sestito LF, Heiler AJ, Schudel A, et al. Drug-eluting immune checkpoint blockade antibody-nanoparticle conjugate enhances locoregional and systemic combination cancer immunotherapy through T lymphocyte targeting. Biomaterials. 2021;279:121184.
Google Scholar
Prakash R, Subbiah V, Iyer SP. Evolving landscape of antibody drug conjugates in lymphoma. Cancer J. 2022;28:479–87.
Google Scholar
Truffi M, Fiandra L, Sorrentino L, Monieri M, Corsi F, Mazzucchelli S. Ferritin nanocages: a biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol Res. 2016;107:57–65.
Google Scholar
Kaur R, Badea I. Nanodiamonds as novel nanomaterials for biomedical applications: drug delivery and imaging systems. Int J Nanomedicine. 2013;8:203–20.
Google Scholar
Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45.
Google Scholar
Ruman U, Fakurazi S, Masarudin MJ, Hussein MZ. Nanocarrier-based therapeutics and theranostics drug delivery systems for next generation of liver cancer nanodrug modalities. Int J Nanomedicine. 2020;15:1437–56.
Google Scholar
Ibsen S, Benchimol M, Simberg D, Schutt C, Steiner J, Esener S. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. J Control Release. 2011;155:358–66.
Google Scholar
Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater. 2009;8:935–9.
Google Scholar
Dey T, Ghosh A, Sanyal A, Charles CJ, Pokharel S, Nair L, et al. Surface engineered nanodiamonds: mechanistic intervention in biomedical applications for diagnosis and treatment of cancer. Biomed Mater. 2024. https://doi.org/10.1088/1748-605X/ad3abb
Gao XJ, Ciura K, Ma Y, Mikolajczyk A, Jagiello K, Wan Y, et al. Toward the integration of machine learning and molecular modeling for designing drug delivery nanocarriers. Adv Mater. 2024;36:e2407793.
Google Scholar
Serov N, Vinogradov V. Artificial intelligence to bring nanomedicine to life. Adv Drug Deliv Rev. 2022;184:114194.
Google Scholar
Lin Z, Chou WC, Cheng YH, He C, Monteiro-Riviere NA, Riviere JE. Predicting nanoparticle delivery to tumors using machine learning and artificial intelligence approaches. Int J Nanomedicine. 2022;17:1365–79.
Google Scholar
Roskopf CC, Braciak TA, Fenn NC, Kobold S, Fey GH, Hopfner KP, et al. Dual-targeting triplebody 33-3-19 mediates selective lysis of biphenotypic CD19+ CD33+ leukemia cells. Oncotarget. 2016;7:22579–89.
Google Scholar
Shramova E, Proshkina G, Shipunova V, Ryabova A, Kamyshinsky R, Konevega A, et al. Dual Targeting of Cancer Cells with DARPin-Based Toxins for Overcoming Tumor Escape. Cancers (Basel). 2020;12:3014.
Wang M, Ma Q, Suthe SR, Hudson RE, Pan JY, Mikelis C, et al. Humanized dual-targeting antibody-drug conjugates specific to MET and RON receptors as a pharmaceutical strategy for the treatment of cancers exhibiting phenotypic heterogeneity. Acta Pharmacol Sin. 2025;46:1375–89.
Google Scholar
Taghipour YD, Zarebkohan A, Salehi R, Rahimi F, Torchilin VP, Hamblin MR, et al. An update on dual targeting strategy for cancer treatment. J Control Release. 2022;349:67–96.
Google Scholar
Tsujii S, Serada S, Fujimoto M, Uemura S, Namikawa T, Nomura T, et al. Glypican-1 is a novel target for stroma and tumor cell dual-targeting antibody-drug conjugates in pancreatic cancer. Mol Cancer Ther. 2021;20:2495–505.
Google Scholar
Yu X, Long Y, Chen B, Tong Y, Shan M, Jia X, et al. PD-L1/TLR7 dual-targeting nanobody-drug conjugate mediates potent tumor regression via elevating tumor immunogenicity in a host-expressed PD-L1 bias-dependent way. J Immunother Cancer. 2022;10:e004590.
Yi T, Yang L, Hao K, Cao W, Qi Y, Gai J, et al. Abstract 4776: preclinical study of bispecific antibody drug conjugates (bsADCs) targeting DLL3 and SEZ6 demonstrated potent anti-tumor activity in small cell lung cancer (SCLC) xenograft models. Cancer Res. 2025;85:4776–4776.
Chai X, Li F, Xie H, Lu Y, Li N, Yu H, et al. Abstract 340: gensci139, a highly differentiated EGFR×HER2 bispecific ADC, for the treatment of multiple solid tumors. Cancer Res. 2025;85:340–340.
Richards D, Braiteh F, Anthony S, Edenfield W, Hellerstedt B, Raju R, et al. 496p – a phase 1 study of MM-111; a bispecific HER2/HER3 antibody fusion protein, combined with multiple treatment regimens in patients with advanced HER2 positive solid tumors. Ann Oncol. 2012;23:ix170.
Guidi L, Etessami J, Valenza C, Valdivia A, Meric-Bernstam F, Felip E, et al. Bispecific antibodies in hematologic and solid tumors: current landscape and therapeutic advances. Am Soc Clin Oncol Educ Book. 2025;45:e473148.
Google Scholar
Herrera M, Pretelli G, Desai J, Garralda E, Siu LL, Steiner TM, et al. Bispecific antibodies: advancing precision oncology. Trends Cancer. 2024;10:893–919.
Google Scholar
Elshiaty M, Schindler H, Christopoulos P. Principles and Current Clinical Landscape of Multispecific Antibodies against Cancer. Int J Mol Sci. 2021;22:5632.
Qi L, Takeda S, Zhang Y, Huang M, Zhou D, Mao Y, et al. Abstract 2955: preclinical development of TJ101, a potent bispecific ADC targeting EGFR and B7–H3 for the treatment of solid cancers. Cancer Res. 2025;85:2955–2955.
Ettorre VM, AlAshqar A, Sethi N, Santin AD. Personalized Treatment in Ovarian Cancer: A Review of Disease Monitoring, Biomarker Expression, and Targeted Treatments for Advanced, Recurrent Ovarian Cancers. Cancers (Basel). 2025;17:1822.
Zhang F, Li S. Antibody-drug conjugates as game changers in bladder cancer: current progress and future directions. Front Immunol. 2025;16:1591191.
Google Scholar
Trapani D, Katrini J, Curigliano G. Unveiling the untapped potential of antibody drug conjugates in precision oncology. JAMA Oncol. 2024;10:563–4.
Google Scholar
Huang Z, Braunstein Z, Chen J, Wei Y, Rao X, Dong L, et al. Precision medicine in rheumatic diseases: unlocking the potential of antibody-drug conjugates. Pharmacol Rev. 2024;76:579–98.
Google Scholar
Vitiello A, Ferrara F, Lasala R, Zovi A. Precision Medicine in the Treatment of Locally Advanced or Metastatic Urothelial Cancer: New Molecular Targets and Pharmacological Therapies. Cancers (Basel). 2022;14:5167.
Bai X, Xu L, Wang Z, Zhuang X, Ning J, Sun Y, et al. Computational-aided rational mutation design of pertuzumab to overcome active HER2 mutation S310F through antibody-drug conjugates. Proc Natl Acad Sci U S A. 2025;122:e2413686122.
Google Scholar
Ascione L, Guidi L, Prakash A, Trapani D, LoRusso P, Lou E, et al. Unlocking the potential: Biomarkers of response to antibody-drug conjugates. Am Soc Clin Oncol Educ Book. 2024;44:e431766.
Google Scholar
Kathad U, Biyani N. Peru YCDPRL, Zhou J, Kochat H, Bhatia K: Expanding the repertoire of Antibody Drug Conjugate (ADC) targets with improved tumor selectivity and range of potent payloads through in-silico analysis. PLoS ONE. 2024;19:e0308604.
Google Scholar
Tagde P, Najda A, Nagpal K, Kulkarni GT, Shah M, Ullah O, et al. Nanomedicine-Based Delivery Strategies for Breast Cancer Treatment and Management. Int J Mol Sci. 2022;23:2856.
Wang Y, Jin RU, Xu J, Lin DC, Sun Z, Xu Y, et al. Harnessing technologies to unravel gastric cancer heterogeneity. Trends Cancer. 2025;11:753-769.
Zhang C, Li N, Zhang P, Jiang Z, Cheng Y, Li H, et al. Advancing precision and personalized breast cancer treatment through multi-omics technologies. Am J Cancer Res. 2024;14:5614–27.
Google Scholar
Cairo S, Gilardi M, Zipeto M, Ritchie M. Abstract 6851: an integrated preclinical platform for antibody-drug conjugate development. Cancer Res. 2025;85:6851–6851.
Stojchevski R, Sutanto EA, Sutanto R, Hadzi-Petrushev N, Mladenov M, Singh SR, et al. Translational Advances in Oncogene and Tumor-Suppressor Gene Research. Cancers (Basel). 2025;17:1008.
Di Sario G, Rossella V, Famulari ES, Maurizio A, Lazarevic D, Giannese F, et al. Enhancing clinical potential of liquid biopsy through a multi-omic approach: a systematic review. Front Genet. 2023;14:1152470.
Google Scholar
Zhang YW, Gvozdenovic A, Aceto N. A molecular voyage: multiomics insights into circulating tumor cells. Cancer Discov. 2024;14:920–33.
Google Scholar
Cela I, Capone E, Pece A, Lovato G, Simeone P, Colasante M, et al. Lgals3bp antibody-drug conjugate enhances tumor-infiltrating lymphocytes and synergizes with immunotherapy to restrain neuroblastoma growth. J Transl Med. 2025;23:431.
Google Scholar
Lu C, Li Z, Wu N, Lu D, Zhang X-B, Song G. Tumor microenvironment-tailored nanoplatform for companion diagnostic applications of precise cancer therapy. Chem. 2023;9:3185–211.
Google Scholar
Puccetti M, Pariano M, Schoubben A, Giovagnoli S, Ricci M. Biologics, theranostics, and personalized medicine in drug delivery systems. Pharmacol Res. 2024;201:107086.
Google Scholar
Vranic S, Gatalica Z. The role of pathology in the era of personalized (precision) medicine: a brief review. Acta Med Acad. 2021;50:47–57.
Google Scholar
Valla V, Alzabin S, Koukoura A, Lewis A, Nielsen AA, Vassiliadis E. Companion diagnostics: state of the art and new regulations. Biomark Insights. 2021;16:11772719211047763.
Google Scholar
Wu J, Zhang Y, Jiang K, Wang X, Blum NT, Zhang J, et al. Enzyme-engineered conjugated polymer nanoplatform for activatable companion diagnostics and multistage augmented synergistic therapy. Adv Mater. 2022;34:e2200062.
Google Scholar
Antonarelli G, Corti C, Tarantino P, Salimbeni BT, Zagami P, Marra A, et al. Management of patients with HER2-positive metastatic breast cancer after trastuzumab deruxtecan failure. ESMO Open. 2023;8:101608.
Google Scholar
Rassy E, Rached L, Pistilli B. Antibody drug conjugates targeting HER2: clinical development in metastatic breast cancer. Breast. 2022;66:217–26.
Google Scholar
Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15:24.
Google Scholar
Xiao D, Luo L, Li J, Wang Z, Liu L, Xie F, et al. Development of bifunctional anti-PD-L1 antibody MMAE conjugate with cytotoxicity and immunostimulation. Bioorg Chem. 2021;116:105366.
Google Scholar
Zhang P, Tao C, Xie H, Yang L, Lu Y, Xi Y, et al. Identification of CD66c as a potential target in gastroesophageal junction cancer for antibody-drug conjugate development. Gastric Cancer. 2025;28:422–41.
Google Scholar
Usama M, Hsu YC, Safaei M, Chen CY, Han KH, Ho YS, et al. Antibody-drug conjugates targeting SSEA-4 inhibits growth and migration of SSEA-4 positive breast cancer cells. Cancer Lett. 2025;611:217453.
Google Scholar
Hao X, Liu J, li h, li j, wang j, Hu H-H, et al. Abstract 4723: development and preclinical activity of BSI-721, a novel antibody-drug conjugate (ADC) targeting cadherin 17 (CDH17) in gastrointestinal cancers. Cancer Res. 2025;85:4723–4723.
Wehn AK, Qiu P, Lunceford J, Yarunin A, Cristescu R, Liu L, et al. Concordance between an FDA-approved companion diagnostic and an alternative assay kit for assessing homologous recombination deficiency in ovarian cancer. Gynecol Oncol. 2024;184:67–73.
Google Scholar
Ohmura H, Hanamura F, Okumura Y, Ando Y, Masuda T, Mimori K, et al. Liquid biopsy for breast cancer and other solid tumors: a review of recent advances. Breast Cancer. 2025;32:33–42.
Google Scholar
Ju Y, Watson J, Wang JJ, Yen YT, Gevorkian L, Chen Z, et al. B7-H3-liquid biopsy for the characterization and monitoring of the dynamic biology of prostate cancer. Drug Resist Updat. 2025;79:101207.
Google Scholar
Ikwelle TA, Ihim AC, Ozuruoke DFN, Obi PC, Obi CU, Onuora IJ, et al. Multi-omics integration in personalized medicine: advancing laboratory diagnostics and precision therapeutics in the era of individualized healthcare. J Drug Deliv Ther. 2025;15:132–42.
Google Scholar
Nicolò E, Giugliano F, Ascione L, Tarantino P, Corti C, Tolaney SM, et al. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treat Rev. 2022;106:102395.
Google Scholar
Klein C, Brinkmann U, Reichert JM, Kontermann RE. The present and future of bispecific antibodies for cancer therapy. Nat Rev Drug Discov. 2024;23:301–19.
Google Scholar
Chandra J, Molugulu N, Gupta G, Siddiqua A, Wahab S, Kesharwani P. Biomimetic nanoparticles: a revolutionary approach to breast cancer therapy using cell membrane coatings. J Drug Deliv Sci Technol. 2025;107:106849.
Google Scholar
Puzzo M, De Santo M, Morelli C, Leggio A, Pasqua L. The advent of molecular targeted therapies against cancer. Toward multi-targeting drugs through materials engineering: a possible future scenario. Small Sci. 2024;4:2400113.
Google Scholar
Kiani MN, Khaliq H, Abubakar M, Rafique M, Jalilov F, Ashraf GA, et al. Advancing the potential of nanoparticles for cancer detection and precision therapeutics. Med Oncol. 2025;42:239.
Google Scholar
Song CH, Jeong M, In H, Kim JH, Lin CW, Han KH. Trends in the Development of Antibody-Drug Conjugates for Cancer Therapy. Antibodies (Basel). 2023;12:72.
Li K, Xie G, Deng X, Zhang Y, Jia Z, Huang Z. Antibody-drug conjugates in urinary tumors: clinical application, challenge, and perspectives. Front Oncol. 2023;13:1259784.
Google Scholar
Kim HI, Park J, Zhu Y, Wang X, Han Y, Zhang D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp Mol Med. 2024;56:836–49.
Google Scholar
Chen Z, Kankala RK, Yang Z, Li W, Xie S, Li H, et al. Antibody-based drug delivery systems for cancer therapy: mechanisms, challenges, and prospects. Theranostics. 2022;12:3719–46.
Google Scholar
Palakurthi SS, Shah B, Kapre S, Charbe N, Immanuel S, Pasham S, et al. A comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv. 2024;6:5803–26.
Google Scholar
Marques AC, Costa PC, Velho S, Amaral MH. Lipid Nanoparticles Functionalized with Antibodies for Anticancer Drug Therapy. Pharmaceutics. 2023;15:216.
Reynolds JG, Geretti E, Hendriks BS, Lee H, Leonard SC, Klinz SG, et al. HER2-targeted liposomal doxorubicin displays enhanced anti-tumorigenic effects without associated cardiotoxicity. Toxicol Appl Pharmacol. 2012;262:1–10.
Google Scholar
Munster P, Krop IE, LoRusso P, Ma C, Siegel BA, Shields AF, et al. Safety and pharmacokinetics of MM-302, a HER2-targeted antibody-liposomal doxorubicin conjugate, in patients with advanced HER2-positive breast cancer: a phase 1 dose-escalation study. Br J Cancer. 2018;119:1086–93.
Google Scholar
Gaddy DF, Lee H, Zheng J, Jaffray DA, Wickham TJ, Hendriks BS. Whole-body organ-level and kidney micro-dosimetric evaluations of (64)Cu-loaded HER2/ErbB2-targeted liposomal doxorubicin ((64)Cu-MM-302) in rodents and primates. EJNMMI Res. 2015;5:24.
Google Scholar
Wiklander OPB, Mamand DR, Mohammad DK, Zheng W, Jawad Wiklander R, Sych T, et al. Antibody-displaying extracellular vesicles for targeted cancer therapy. Nat Biomed Eng. 2024;8:1453–68.
Google Scholar
Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. Exosomes─nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. 2022;16:17802–46.
Google Scholar
Zheng Y, Hasan A, Nejadi Babadaei MM, Behzadi E, Nouri M, Sharifi M, et al. Exosomes: multiple-targeted multifunctional biological nanoparticles in the diagnosis, drug delivery, and imaging of cancer cells. Biomed Pharmacother. 2020;129:110442.
Google Scholar
Li X, Peng X, Zoulikha M, Boafo GF, Magar KT, Ju Y, et al. Multifunctional nanoparticle-mediated combining therapy for human diseases. Signal Transduct Target Ther. 2024;9:1.
Google Scholar
Zhang M, Hu S, Liu L, Dang P, Liu Y, Sun Z, et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8:124.
Google Scholar
Miceli RT, Chen TY, Nose Y, Tichkule S, Brown B, Fullard JF, et al. Extracellular vesicles, RNA sequencing, and bioinformatic analyses: challenges, solutions, and recommendations. J Extracell Vesicles. 2024;13:e70005.
Google Scholar
Verma N, Arora S. Navigating the Global Regulatory Landscape for Exosome-Based Therapeutics: Challenges, Strategies, and Future Directions. Pharmaceutics. 2025;17:990.
Miller K, Cortes J, Hurvitz SA, Krop IE, Tripathy D, Verma S, et al. HERMIONE: a randomized phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer. 2016;16:352.
Google Scholar
Ojima I. Guided molecular missiles for tumor-targeting chemotherapy–case studies using the second-generation taxoids as warheads. Acc Chem Res. 2008;41:108–19.
Google Scholar
Khan KA, Kerbel RS. A CD276 antibody guided missile with one warhead and two targets: the tumor and its vasculature. Cancer Cell. 2017;31:469–71.
Google Scholar
Chis AA, Dobrea CM, Arseniu AM, Frum A, Rus LL, Cormos G, et al. Antibody-Drug Conjugates-Evolution and Perspectives. Int J Mol Sci. 2024;25:6969.
Mahalingaiah PK, Ciurlionis R, Durbin KR, Yeager RL, Philip BK, Bawa B, et al. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol Ther. 2019;200:110–25.
Google Scholar
Fatima SW, Khare SK. Benefits and challenges of antibody drug conjugates as novel form of chemotherapy. J Control Release. 2022;341:555–65.
Google Scholar
Paz-Manrique R, Pinto JA, Gomez Moreno HL. Antibody-drug conjugates in breast cancer: toward a molecular perspective into clinical practice. JCO Precis Oncol. 2024;8:e2400173.
Google Scholar
Felber JG, Thorn-Seshold O. 40 years of duocarmycins: a graphical structure/function review of their chemical evolution, from SAR to prodrugs and ADCs. JACS Au. 2022;2:2636–44.
Google Scholar
Dong W, Wang W, Cao C. The evolution of antibody-drug conjugates: toward accurate DAR and multi-specificity. ChemMedChem. 2024;19:e202400109.
Google Scholar
Ter Heine R, van den Heuvel MM, Piet B, Deenen MJ, van der Wekken AJ, Hendriks LEL, et al. A Systematic Evaluation of Cost-Saving Dosing Regimens for Therapeutic Antibodies and Antibody-Drug Conjugates for the Treatment of Lung Cancer. Target Oncol. 2023;18:441–50.
Google Scholar
Walsh SJ, Bargh JD, Dannheim FM, Hanby AR, Seki H, Counsell AJ, et al. Site-selective modification strategies in antibody-drug conjugates. Chem Soc Rev. 2021;50:1305–53.
Google Scholar
Behrens CR, Liu B. Methods for site-specific drug conjugation to antibodies. MAbs. 2014;6:46–53.
Google Scholar
Evans N, Grygorash R, Williams P, Kyle A, Kantner T, Pathak R, et al. Incorporation of hydrophilic macrocycles into drug-linker reagents produces antibody-drug conjugates with enhanced in vivo performance. Front Pharmacol. 2022;13:764540.
Google Scholar
Lyon RP, Bovee TD, Doronina SO, Burke PJ, Hunter JH, Neff-LaFord HD, et al. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat Biotechnol. 2015;33:733–5.
Google Scholar
Bardia A, Hu X, Dent R, Yonemori K, Barrios CH, O’Shaughnessy JA, et al. Trastuzumab deruxtecan after endocrine therapy in metastatic breast cancer. N Engl J Med. 2024;391:2110–22.
Google Scholar
Whitehorn A, Fu L, Porritt K, Lizarondo L, Stephenson M, Marin T, et al. Mapping clinical barriers and evidence-based implementation strategies in low-to-middle income countries (LMICs). Worldviews Evid Based Nurs. 2021;18:190–200.
Google Scholar
Osunkwo I, James J, El-Rassi F, Nero A, Minniti CP, Trimnell C, et al. Burden of disease, treatment utilization, and the impact on education and employment in patients with sickle cell disease: a comparative analysis of high- and low- to middle-income countries for the international Sickle Cell World Assessment Survey. Am J Hematol. 2022;97:1055–64.
Google Scholar
Cauchon NS, Oghamian S, Hassanpour S, Abernathy M. Innovation in chemistry, manufacturing, and controls-a regulatory perspective from industry. J Pharm Sci. 2019;108:2207–37.
Google Scholar
Raja A, Kasana A, Verma V. Next-Generation Therapeutic Antibodies for Cancer Treatment: Advancements, Applications, and Challenges. Mol Biotechnol. 2025;67:3345-3365.
Tolcher A, Hamilton E, Coleman RL. The evolving landscape of antibody-drug conjugates in gynecologic cancers. Cancer Treat Rev. 2023;116:102546.
Google Scholar
Schmitt S, Machui P, Mai I, Herterich S, Wunder S, Cyprys P, et al. Design and evaluation of phosphonamidate-linked exatecan constructs for highly loaded, stable, and efficacious antibody-drug conjugates. Mol Cancer Ther. 2024;23:199–211.
Google Scholar
Neff-LaFord HD, Carratt SA, Carosino C, Everds N, Cardinal KA, Duniho S, et al. Zuch de Zafra C, Harstad EB: The Vedotin Antibody-Drug Conjugate Payload Drives Platform-Based Nonclinical Safety and Pharmacokinetic Profiles. Mol Cancer Ther. 2024;23:1483–93.
Google Scholar
Toader D, Fessler SP, Collins SD, Conlon PR, Bollu R, Catcott KC, et al. Discovery and preclinical characterization of XMT-1660, an optimized B7-H4-targeted antibody-drug conjugate for the treatment of cancer. Mol Cancer Ther. 2023;22:999–1012.
Google Scholar
Liu KF, Liu YX, Dai L, Li CX, Wang L, Liu J, et al. A novel self-assembled pH-sensitive targeted nanoparticle platform based on antibody-4arm-polyethylene glycol-pterostilbene conjugates for co-delivery of anticancer drugs. J Mater Chem B. 2018;6:656–65.
Google Scholar
Hammood M, Craig AW, Leyton JV. Impact of Endocytosis Mechanisms for the Receptors Targeted by the Currently Approved Antibody-Drug Conjugates (ADCs)-A Necessity for Future ADC Research and Development. Pharmaceuticals (Basel). 2021;14:674.
Abdollahpour-Alitappeh M, Lotfinia M, Gharibi T, Mardaneh J, Farhadihosseinabadi B, Larki P, et al. Antibody-drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J Cell Physiol. 2019;234:5628–42.
Google Scholar
Durbin KR, Phipps C, Liao X. Mechanistic modeling of antibody-drug conjugate internalization at the cellular level reveals inefficient processing steps. Mol Cancer Ther. 2018;17:1341–51.
Google Scholar
Dela Cruz Chuh J, Go M, Chen Y, Guo J, Rafidi H, Mandikian D, et al. Preclinical optimization of Ly6E-targeted ADCs for increased durability and efficacy of anti-tumor response. MAbs. 2021;13:1862452.
Google Scholar
Xu S. Internalization, trafficking, intracellular processing and actions of antibody-drug conjugates. Pharm Res. 2015;32:3577–83.
Google Scholar
Pegram MD, Miles D, Tsui CK, Zong Y. HER2-overexpressing/amplified breast cancer as a testing ground for antibody-drug conjugate drug development in solid tumors. Clin Cancer Res. 2020;26:775–86.
Google Scholar
Goldmacher VS, Gershteyn IM, Kovtun Y. Beyond ADCs: harnessing bispecific antibodies to directly induce apoptosis for targeted tumor eradication. Antibody Therapeutics. 2024;7:351–60.
Google Scholar
Gouda MA, Gonugunta A, Dumbrava EE, Yap TA, Rodon J, Piha-Paul SA, et al. Human epidermal growth factor receptor 2 loss following treatment with Trastuzumab Deruxtecan in patients with metastatic breast cancer. Clin Cancer Res. 2025;31:1268–74.
Google Scholar
von Arx C, De Placido P, Caltavituro A, Di Rienzo R, Buonaiuto R, De Laurentiis M, et al. The evolving therapeutic landscape of trastuzumab-drug conjugates: future perspectives beyond HER2-positive breast cancer. Cancer Treat Rev. 2023;113:102500.
Pereslete AM, Hughes ME, Martin AR, Files J, Nguyen K, Buckley L, et al. Analysis of HER2 expression changes from breast primary to brain metastases and the impact of HER2-low expression on overall survival. Neuro Oncol. 2025;27:184–94.
Google Scholar
Bon G, Pizzuti L, Laquintana V, Loria R, Porru M, Marchiò C, et al. Loss of HER2 and decreased T-DM1 efficacy in HER2 positive advanced breast cancer treated with dual HER2 blockade: the sePHER study. J Exp Clin Cancer Res. 2020;39:279.
Google Scholar
Nicolò E, Boscolo Bielo L, Curigliano G, Tarantino P. The HER2-low revolution in breast oncology: steps forward and emerging challenges. Ther Adv Med Oncol. 2023;15:17588359231152842.
Google Scholar
Wang JQ, Teng QX, Lei ZN, Ji N, Cui Q, Fu H, et al. Reversal of cancer multidrug resistance (MDR) mediated by ATP-binding cassette transporter G2 (ABCG2) by AZ-628, a RAF kinase inhibitor. Front Cell Dev Biol. 2020;8:601400.
Google Scholar
Chen R, Herrera AF, Hou J, Chen L, Wu J, Guo Y, Synold TW, Ngo VN, Puverel S, Mei M, et al: Inhibition of MDR1 Overcomes Resistance to Brentuximab Vedotin in Hodgkin Lymphoma. Clin Cancer Res 2020, 26:1034–1044.
Chang CH, Wang Y, Zalath M, Liu D, Cardillo TM, Goldenberg DM. Combining ABCG2 inhibitors with IMMU-132, an anti-Trop-2 antibody conjugate of SN-38, overcomes resistance to SN-38 in breast and gastric cancers. Mol Cancer Ther. 2016;15:1910–9.
Google Scholar
Patnaik SK, Chandrasekar MJN, Nagarjuna P, Ramamurthi D, Swaroop AK. Targeting of ErbB1, ErbB2, and their dual targeting using small molecules and natural peptides: blocking EGFR cell signaling pathways in cancer: a mini-review. Mini Rev Med Chem. 2022;22:2831–46.
Google Scholar
Morchón-Araujo D, Catani G, Mirallas O, Pretelli G, Sánchez-Pérez V, Vieito M, et al. Emerging Immunotherapy Targets in Early Drug Development. Int J Mol Sci. 2025;26:5394.
Ackerman SE, Pearson CI, Gregorio JD, Gonzalez JC, Kenkel JA, Hartmann FJ, et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat Cancer. 2021;2:18–33.
Google Scholar
Slezak A, Chang K, Hossainy S, Mansurov A, Rowan SJ, Hubbell JA, et al. Therapeutic synthetic and natural materials for immunoengineering. Chem Soc Rev. 2024;53:1789–1822.
Google Scholar
Lei Y, Liu J, Bai Y, Zheng C, Wang D. Peptides as Versatile Regulators in Cancer Immunotherapy: Recent Advances, Challenges, and Future Prospects. Pharmaceutics. 2025;17:46.
Wang L, Ke Y, He Q, Paerhati P, Zhuang W, Yue Y, et al. A novel ROR1-targeting antibody-PROTAC conjugate promotes BRD4 degradation for solid tumor treatment. Theranostics. 2025;15:1238–54.
Google Scholar
Wang C, Zhang Y, Chen W, Wu Y, Xing D. New-generation advanced PROTACs as potential therapeutic agents in cancer therapy. Mol Cancer. 2024;23:110.
Google Scholar
Salerno A, Seghetti F, Caciolla J, Uliassi E, Testi E, Guardigni M, et al. Enriching proteolysis targeting chimeras with a second modality: when two are better than one. J Med Chem. 2022;65:9507–30.
Google Scholar
Kumari S, Raj S, Babu MA, Bhatti GK, Bhatti JS. Antibody-drug conjugates in cancer therapy: innovations, challenges, and future directions. Arch Pharm Res. 2024;47:40–65.
Google Scholar
Ghose A, Lapitan P, Apte V, Ghosh A, Kandala A, Basu S, et al. Antibody drug conjugates in urological cancers: a review of the current landscape. Curr Oncol Rep. 2024;26:633–46.
Google Scholar
Justiz-Vaillant A, Pandit BR, Unakal C, Vuma S, Akpaka PE. A Comprehensive Review About the Use of Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel). 2025;14:35.
Li S, Zhao X, Fu K, Zhu S, Pan C, Yang C, et al. Resistance to antibody-drug conjugates: a review. Acta Pharm Sin B. 2025;15:737–56.
Google Scholar
Chen YF, Xu YY, Shao ZM, Yu KD. Resistance to antibody-drug conjugates in breast cancer: mechanisms and solutions. Cancer Commun. 2023;43(3):297–337.
Restelli C, Ruella M, Paruzzo L, Tarella C, Pelicci PG, Colombo E. Recent advances in immune-based therapies for acute myeloid leukemia. Blood Cancer Discov. 2024;5:234–48.
Google Scholar
Jabbarzadeh Kaboli P, Roozitalab G, Farghadani R, Eskandarian Z, Zerrouqi A. C-MET and the immunological landscape of cancer: novel therapeutic strategies for enhanced anti-tumor immunity. Front Immunol. 2024;15:1498391.
Google Scholar
Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–22.
Google Scholar
Cipriano É, Mesquita A. Emerging therapeutic drugs in metastatic triple-negative breast cancer. Breast Cancer (Auckl). 2021;15:11782234211002491.
Google Scholar
Parneet C, Sylvia H, Marianna K, Maximilian H, Frances AS, Quincy C, et al. 695 Efficacy and safety of trastuzumab deruxtecan (T-DXd) with durvalumab in patients with non-small cell lung cancer (HER2 altered NSCLC) who progressed on anti-PD1/PD-L1 therapy (HUDSON). J Immunother Cancer. 2023;11:787.
Shi Z, Lu Y, Zhao Q, Wang Y, Qiu P. Antibody-drug conjugates in breast cancer: advances and prospects. Cancer Biol Med. 2025;22:83–92.
Google Scholar
Wang Y. Durvalumab and T-DXd synergistically promote apoptosis of cholangiocarcinoma cells by downregulating EGR1 expression through inhibiting P38 MAPK pathway. Appl Biochem Biotechnol. 2025;197:1773–89.
Google Scholar
Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381:e071674.
Google Scholar
Khosravanian MJ, Mirzaei Y, Mer AH, Keyhani-Khankahdani M, Abdinia FS, Misamogooe F, et al. Nectin-4-directed antibody-drug conjugates (ADCs): spotlight on preclinical and clinical evidence. Life Sci. 2024;352:122910.
Google Scholar
Sflakidou E, Leonidis G, Foroglou E, Siokatas C, Sarli V. Recent Advances in Natural Product-Based Hybrids as Anti-Cancer Agents. Molecules. 2022;27:6632.
Wong JL, Rosenberg JE. Targeting nectin-4 by antibody-drug conjugates for the treatment of urothelial carcinoma. Expert Opin Biol Ther. 2021;21:863–73.
Google Scholar
Rani B, Ignatz-Hoover JJ, Rana PS, Driscoll JJ. Current and Emerging Strategies to Treat Urothelial Carcinoma. Cancers (Basel). 2023;15:4886.
Tsang ES, Dhawan MS, Pacaud R, Thomas S, Grabowsky J, Wilch L, et al. Synthetic Lethality Beyond BRCA: A Phase I Study of Rucaparib and Irinotecan in Metastatic Solid Tumors With Homologous Recombination-Deficiency Mutations Beyond BRCA1/2. JCO Precis Oncol. 2024;8:e2300494.
Google Scholar
Luo L, Keyomarsi K. PARP inhibitors as single agents and in combination therapy: the most promising treatment strategies in clinical trials for BRCA-mutant ovarian and triple-negative breast cancers. Expert Opin Investig Drugs. 2022;31:607–31.
Google Scholar
Mariappan L, Jiang XY, Jackson J, Drew Y. Emerging treatment options for ovarian cancer: focus on rucaparib. Int J Womens Health. 2017;9:913–24.
Google Scholar
Morganti S, Marra A, De Angelis C, Toss A, Licata L, Giugliano F, et al. PARP inhibitors for breast cancer treatment: a review. JAMA Oncol. 2024;10:658–70.
Google Scholar
Tao J, Gu Y, Zhou W, Wang Y. Dual-payload antibody-drug conjugates: taking a dual shot. Eur J Med Chem. 2025;281:116995.
Google Scholar
Wen M, Yu A, Park Y, Calarese D, Gerber HP, Yin G. Homogeneous antibody-drug conjugates with dual payloads: potential, methods and considerations. MAbs. 2025;17:2498162.
Google Scholar
Hoffmann RM, Mele S, Cheung A, Larcombe-Young D, Bucaite G, Sachouli E, et al. Rapid conjugation of antibodies to toxins to select candidates for the development of anticancer Antibody-drug conjugates (ADCs). Sci Rep. 2020;10:8869.
Google Scholar
Zemek RM, Anagnostou V. Pires da Silva I, Long GV, Lesterhuis WJ: Exploiting temporal aspects of cancer immunotherapy. Nat Rev Cancer. 2024;24:480–97.
Google Scholar
Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–62.
Google Scholar
Bardia A, Viale G. HER2-low breast cancer-diagnostic challenges and opportunities for insights from ongoing studies: a podcast. Target Oncol. 2023;18:313–9.
Google Scholar
Xie S, Wang Y, Gong Z, Li Y, Yang W, Liu G, et al. Liquid biopsy and tissue biopsy comparison with digital PCR and IHC/FISH for HER2 amplification detection in breast cancer patients. J Cancer. 2022;13:744–51.
Google Scholar
Jauw Y, O’Donoghue J, Zijlstra J, Hoekstra O, Menke-van der Houven van Oordt C, Morschhauser F, Carrasquillo J, Zweegman S, Pandit-Taskar N, Lammertsma A, et al: 89 Zr-Immuno-PET: Toward a Noninvasive Clinical Tool to Measure Target Engagement of Therapeutic Antibodies In Vivo. Journal of Nuclear Medicine 2019, 60:jnumed.118.224568.
Ajay A, Wang H, Rezvani A, Savari O, Grubb BJ, McColl KS, et al. Assessment of targets of antibody drug conjugates in SCLC. NPJ Precis Oncol. 2025;9:1.
Google Scholar
Yao Z, Wei G, Song P, Li C, Wang G, Wen Z, et al. Trop2, Nectin-4, and PD-L1 expression profiles in esophageal squamous cell carcinoma: implications for combined immunotherapy and ADC targeted therapies. Pathol Res Pract. 2025;271:156032.
Google Scholar
Dernbach G, Eich ML, Dragomir MP, Anders P, Jurczok N, Stief C, et al. Spatial expression of HER2, NECTIN4, and TROP-2 in muscle-invasive bladder cancer and metastases: implications for pathological and clinical management. Mod Pathol. 2025;38:100753.
Google Scholar
Coy S, Lee JS, Chan SJ, Woo T, Jones J, Alexandrescu S, et al. Systematic characterization of antibody-drug conjugate targets in central nervous system tumors. Neuro Oncol. 2024;26:458–72.
Google Scholar
Feng Y, Lee J, Yang L, Hilton MB, Morris K, Seaman S, et al. Engineering CD276/B7-H3-targeted antibody-drug conjugates with enhanced cancer-eradicating capability. Cell Rep. 2023;42:113503.
Google Scholar
Mayer KE, Mall S, Yusufi N, Gosmann D, Steiger K, Russelli L, et al. T-cell functionality testing is highly relevant to developing novel immuno-tracers monitoring T cells in the context of immunotherapies and revealed CD7 as an attractive target. Theranostics. 2018;8:6070–87.
Google Scholar
Arora R, Cao C, Kumar M, Sinha S, Chanda A, McNeil R, et al. Spatial transcriptomics reveals distinct and conserved tumor core and edge architectures that predict survival and targeted therapy response. Nat Commun. 2023;14:5029.
Google Scholar
Liu YM, Ge JY, Chen YF, Liu T, Chen L, Liu CC, et al. Combined single-cell and spatial transcriptomics reveal the metabolic evolvement of breast cancer during early dissemination. Adv Sci (Weinh). 2023;10:e2205395.
Google Scholar
Nikanjam M, Kato S, Kurzrock R. Liquid biopsy: current technology and clinical applications. J Hematol Oncol. 2022;15:131.
Google Scholar
Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20:71–88.
Google Scholar
Yan F, Rinn KJ, Kullnat JA, Wu AY, Ennett MD, Scott EL, et al. Response of leptomeningeal metastasis of breast cancer with a HER2/neu activating variant to tucatinib: a case report. J Natl Compr Canc Netw. 2022;20:745–52.
Google Scholar
Paul ED, Huraiová B, Valková N, Matyasovska N, Gábrišová D, Gubová S, et al. The spatially informed mFISHseq assay resolves biomarker discordance and predicts treatment response in breast cancer. Nat Commun. 2025;16:226.
Google Scholar