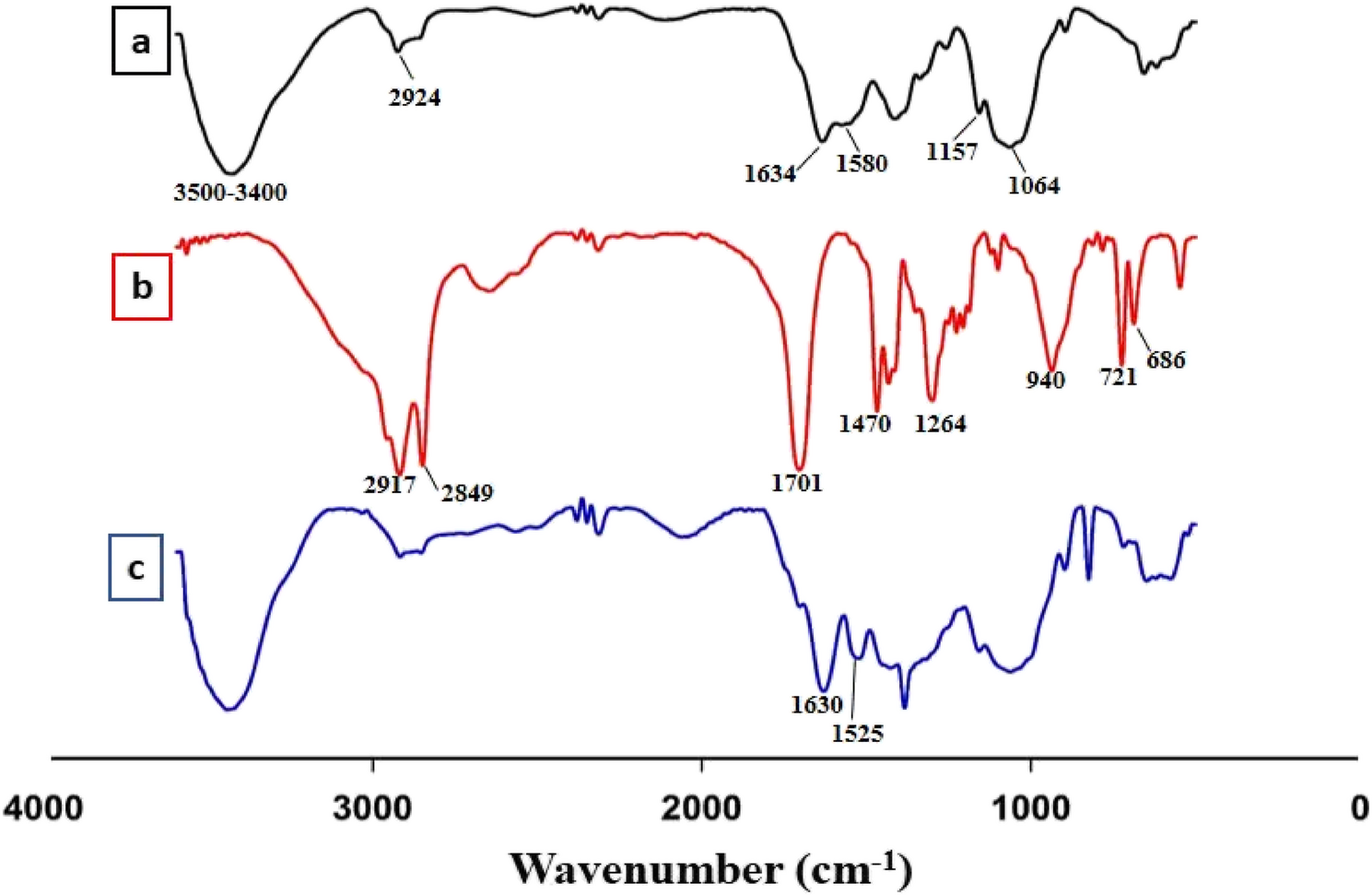
Nanogel chitosan characterization
Fourier-transform infrared spectroscopy (FTIR)
In this study, the nanogel was initially synthesized using modified chitosan. To achieve this modification, a portion of the free amine groups in chitosan react with stearate esters to form amide bonds, which can enhance the properties of chitosan, such as its solubility and bioactivity via the intermediate coupling agent 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC). The conjugation was performed at different molar ratios to assess the reaction efficiency. To confirm the formation of a chemical bond between the amine groups of chitosan and the carboxyl groups of stearate ester, Fourier Transform Infrared Spectroscopy (FT-IR) was employed. Figure 1 presents the FT-IR spectra of pure chitosan, stearate ester, and the resulting nanogels synthesized through the chitosan-stearate ester linkage.
Figure 1a presents the FT-IR spectrum of chitosan. A broad absorption band is observed in the range of 3400–3500 cm⁻¹, which is attributed to the hydroxyl (–OH) groups present in the chitosan structure. At around 1580 cm⁻¹, a peak appears that corresponds to bending vibrations of amide and amine bonds. The peak observed at 2924 cm⁻¹ is related to C–H stretching vibrations, indicating the presence of methyl (–CH₃) and methylene (–CH₂) groups in the chitosan backbone. A distinct peak at 1634 cm⁻¹ is assigned to the stretching vibrations of the amide carbonyl group (C = O), which is associated with the non-deacetylated amine groups. In the region around 1157 cm⁻¹, an asymmetric stretching band related to the ether group (C–O–C) is detected, which is a characteristic feature of chitosan’s molecular structure. Finally, a band at 1064 cm⁻¹ is observed, representing the C–O stretching vibrations, which are also commonly found in the chitosan structure. These findings are consistent with previous reports by Chiono et al. and Peng et al. [24, 25].
Figure 1b displays the FT-IR spectrum of stearate ester. In this spectrum, distinct peaks appear at 2917 cm⁻¹ and 2849 cm⁻¹, which correspond to the stretching vibrations of C–H bonds. The peak observed at 1701 cm⁻¹ is attributed to the stretching vibration of the carbonyl group (C = O) present in the stearate ester structure. Additionally, a peak at 1470 cm⁻¹ is associated with the bending vibration of C–H bonds. In the region of 1261 cm⁻¹, a peak corresponding to the stretching vibrations of the C–O group is observed. The peak at 940 cm⁻¹ indicates the bending vibrations of hydroxyl groups (O–H). Finally, two additional peaks at 721 cm⁻¹ and 686 cm⁻¹ are related to the bending vibrations of C–H bonds within the long hydrocarbon chain of stearate ester.
Figure 1c presents the FT-IR spectrum of the chitosan–stearate ester nanogel. When compared to the spectrum of pure chitosan, notable changes in the intensity of several peaks can be observed. Specifically, the peak intensity around 1630 cm⁻¹, which corresponds to the carbonyl (C = O) group, is markedly increased in the chitosan–stearate ester nanogel. In contrast, the peak at 1525 cm⁻¹, attributed to the free amine (–NH₂) groups, also exhibits changes in intensity. The increased ratio of the C = O peak intensity to that of NH₂ suggests the formation of a higher number of amide bonds in the final nanogel structure. These alterations in peak intensities provide strong evidence for a successful reaction between the amine groups of chitosan and the carboxyl groups of stearate ester. Similar findings have been reported in previous studies, including those conducted by Guo et al., Hadian et al., Waldron et al., and Wang et al. [26,27,28,29].
FTIR spectra of chitosan a stearate ester b and nongel-chitosan/stearate ester c
SEM analysis of chitosan–stearate ester nanogel
Figure 2 displays the scanning electron microscopy (SEM) image of the nanogels synthesized from chitosan and stearate ester. According to this image, the nanogels exhibit a predominantly spherical morphology, with particle sizes estimated to be less than 100 nanometers. This spherical structure forms when the hydrophobic fatty acid stearate ester is incorporated into the chitosan polymer. In a polar medium, this combination tends to self-assemble into micelle-like spherical structures, where the hydrophobic tails orient inward while the hydrophilic heads face outward.
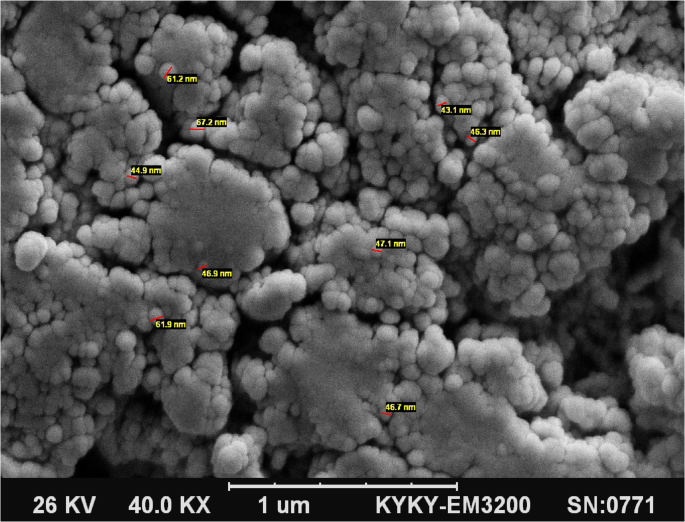
The scanning electron microscopy (SEM) image of the chitosan– stearate ester nanogel containing thyme essential oil
As a biocompatible polymer, chitosan does not trigger allergic responses, and its metabolic byproducts, including amino sugars, are non-toxic and fully absorbed by the body. Numerous studies have explored chitosan-based nanogels formulated with various cross-linking agents. For instance, Nasti et al. developed chitosan nanoparticles with sizes ranging from 160 to 260 nm using tripolyphosphate as a cross-linker [30]. In another study, Lee et al. prepared nanogels of approximately 200 nm in size from a combination of chitosan and glycol [31]. They emphasized that achieving a balance between hydrophobic and hydrophilic interactions is critical for micelle formation, which serves as the primary mechanism behind the nanostructure’s self-assembly. Previous findings have demonstrated that reducing particle size enhances the efficiency of coating materials. Moreover, the use of smaller particles in fruit coatings leads to a reduction in the diameter of surface stomatal pores. This, in turn, decreases the permeability to oxygen and water vapor, effectively limiting enzymatic activity and delaying the onset of fruit spoilage [32].
TEO release from chitosan-based nanogel encapsulation
Evaluating the release profile of encapsulated essential oil is critical to determine whether the chitosan nanogel delivers thymol essential oil in a rapid (burst release) or gradual (sustained release) manner to the fruit matrix. To this end, a spectrophotometric method was employed to monitor the release of thymol essential oil from the chitosan capsules. The release behavior of thymol essential oil from chitosan–stearate ester nanogels under ambient temperature conditions (as shown in Fig. 3) exhibited a biphasic pattern. An initial burst release phase was observed up to day 2, followed by a sustained release phase lasting until day 10. Subsequently, a second burst release phase occurred until day 16, after which a second sustained release phase continued until the end of day 28.

Kinetics of thyme essential oil (TEO) release from chitosan– stearate ester nanogels
The nanogel developed in this study, due to its amphiphilic structure comprising polar (chitosan region) and nonpolar (stearate ester chains) domains, provided an appropriate matrix for the gradual release of the hydrophobic constituents of thyme essential oil into the surrounding environment. This structural characteristic facilitated the slow and controlled release of the active compounds. These findings align with Mohammadi et al. [33], who demonstrated the controlled release of Shirazi thyme essential oil from encapsulated delivery systems. Similarly, Abdalla et al. [34] observed a biphasic release pattern for lemon essential oil encapsulated in pectin–chitosan matrices, consisting of an initial burst followed by sustained release.
Weight loss
Statistical analysis revealed significant differences (p < 0.05) in fruit weight loss during the storage period as influenced by the applied treatments. Although weight loss was observed across all treatments and the control over time, the control group exhibited a markedly higher reduction from day 8 onward compared to the treated samples. All treatment groups effectively delayed weight loss throughout the storage period (Fig. 4).
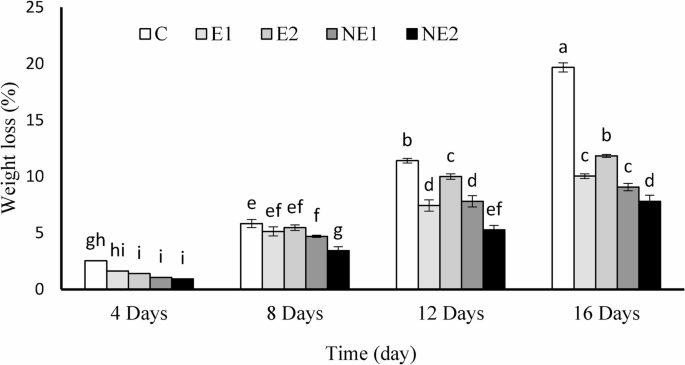
Effects of encapsulated thyme essential oil on the weight loss of strawberry fruits during 16 days of storage at 4 °C. Data are presented as means ± standard deviation (n = 3). Treatments: C (control), E1 (thyme essential oil coating, 600 µL/L), E2 (thyme essential oil coating, 1200 µL/L), NE1 (encapsulated thyme essential oil coating, 600 µL/L), and NE2 (encapsulated thyme essential oil coating, 1200 µL/L)
Interestingly, the application of the essential oil coating at lower concentrations resulted in a more favorable reduction in weight loss. In contrast, higher concentrations of the free essential oil led to increased weight loss. However, encapsulation of the essential oil using chitosan altered this trend; the highest concentration of encapsulated essential oil resulted in the lowest weight loss during storage. By the end of the storage period (day 16), the control fruits had lost 19.66% of their initial weight, while the corresponding weight loss for the E1, E2, NE1, and NE2 treatments was 10.03%, 11.83%, 9.06%, and 7.8%, respectively.
The primary causes of fruit weight loss appear to be moisture transfer from the fruit tissue to the surrounding environment [35] and increased respiration intensity [36]. Additionally, the reduced weight loss observed in fruits coated with thyme essential oil may be attributed to stomatal blockage, which ultimately slows down active metabolic processes and respiration rates. The decrease in weight loss in thyme oil-treated fruits may also result from the semi-permeable nature of the thyme oil coating, which affects moisture loss, respiration, and solute transport across membranes [37]. From day 8 onward, treatments containing higher concentrations of free essential oil exhibited increased weight loss, possibly due to greater evaporation of the essential oil over prolonged storage. The volatile nature of the essential oil may alter the fruit’s tissue structure and molecular integrity, a phenomenon previously reported during investigations of different concentrations of thyme essential oil on strawberry shelf life [37]. Encapsulation of thyme essential oil within chitosan significantly reduced its evaporation rate and prolonged its effectiveness in preserving tissue moisture content by limiting gas exchange between the atmosphere and the fruit tissue. Consequently, unlike the free essential oil treatment at higher concentration (E2), the most pronounced effect was observed in the corresponding encapsulated treatment (NE2). Reduced water loss following the application of plant essential oils and nano-encapsulated formulations has also been reported in sweet marjoram [38], as well as in other horticultural crops such as strawberries [39], wine grapes [40], and bell peppers [37, 41]. One of the advantages of using nanoparticles lies in their high surface-area-to-volume ratio, which enhances reactivity and cellular penetration [42]. Therefore, nanoencapsulation of thyme essential oil using chitosan in the present study effectively contributed to preserving the fresh weight of strawberries over the storage period.
Firmness
The effect of thyme essential oil (E1 and E2) and nano-encapsulated thyme essential oil (NE1 and NE2) in chitosan-based nanogels on the firmness of strawberry fruit during storage is presented in Fig. 5. Regardless of the treatment type, fruit firmness declined over the storage period, decreasing from an initial value of 2.66 N at the beginning of the experiment. Over the 16-day storage period, the reduction in firmness for the coating treatments E1, E2, NE1, and NE2 was 58.47%, 37%, 23%, and 9.06%, respectively. In contrast, the control group exhibited a significantly greater firmness loss of 66%, particularly when compared to the NE2 treatments. The highest firmness retention was observed in the NE2 treatment (2.03 N), followed by NE1 (1.66 N).
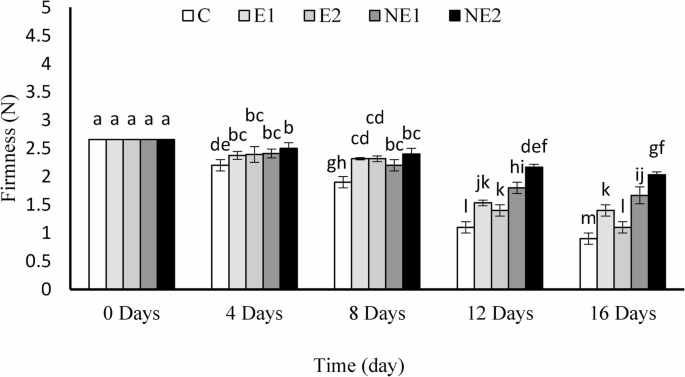
Effects of encapsulated thyme essential oil on the firmness of strawberry fruits during 16 days of storage at 4 °C. Data were represented as means ± standard deviation (n = 3). Treatments: C (control), E1 (thyme essential oil coating 600 µL/L), E2 (thyme essential oil coating 1200 µL/L), NE1 (encapsulated thyme essential oil coating 600 µL/L) and NE2 (encapsulated thyme essential oil coating 1200 µL/L)
The notable reduction in firmness is primarily attributed to tissue water loss and the activation of cell wall–degrading enzymes such as pectin methyl esterase and polygalacturonase [43]. The reduced weight loss observed in the TEO and NE-TEO treatments highlights the efficacy of these coatings in limiting moisture loss and consequently slowing down respiratory metabolism in strawberries. Moreover, the gradual release of TEO from the chitosan nanogel matrix likely disrupted enzyme activity, particularly those involved in cell wall softening, thereby reducing the rate of pectin hydrolysis and contributing to better firmness retention. These findings align with previous reports showing that coatings enriched with citrus essential oils reduced metabolic rates and preserved the firmness of strawberries by inhibiting enzymes responsible for pectin degradation [43]. Furthermore, a recent study by Li et al. [44] demonstrated that nanoencapsulation of thyme essential oil using chitosan cross-linked with sodium tripolyphosphate (TPP) preserved strawberry firmness over 7 days of storage at 25 °C. In contrast, this study used stearate ester as the cross-linking agent in chitosan–thyme oil nanogels and observed substantial firmness retention over an extended 16-day storage period at 4 °C.
Total soluble solids (TSS), titratable acidity (TA), and pH
The changes in TSS content in control and treated strawberry fruits during storage are presented in Fig. 6a. The most pronounced variation in TSS was observed in the control group, followed by the treatment with a higher concentration of free essential oil (E2). In these two treatments, TSS levels initially increased by day 4, followed by a sharp decline from day 8 until the end of the storage period, compared to the other treatments. The remaining treatments exhibited a similar trend, though the changes were less intense and progressed more gradually. Specifically, the highest TSS values in the NE2, NE1, and E1 treatments decreased from an initial 7.2 °Brix at the start of storage to 7.13, 6.53, and 6.56 °Brix, respectively, by the end of the storage period.
The observed increase in TSS accumulation in fruits coated with nano-encapsulated thyme essential oil in chitosan nanogels may be attributed to a reduction in respiratory rate and overall metabolic activity, resulting in lower consumption of sugars through these pathways. Furthermore, interactions between the essential oil and cellular membranes may influence the fruit’s metabolic pathways and senescence processes. Moreover, interactions between the essential oil and cellular membranes may influence the fruit’s metabolic pathways and senescence process. The observed reduction in TSS content at higher concentrations of free essential oil indicates a potential adverse effect on fruit tissue, possibly accelerating ripening or senescence, consistent with previous reports [9]. Additionally, Velichkova et al. [45] reported that reduced weight loss may lead to an apparent increase in sugar concentration due to lower water loss, while decreased decay incidence can limit sugar consumption by fungal pathogens.
According to Fig. 6b, the trend of changes in titratable acidity (TA) of strawberry fruit juice during storage showed a general decline from day 4 until the end of the storage period, except for the control fruits, which exhibited an increasing trend from day 12 to day 16. The most minor in TA was observed in the NE2 and NE1 treatments, where acidity levels decreased from an initial 5.8% to 5.09% and 4.32%, respectively, by day 16. In contrast, the TA in the control group decreased from 5.8% at the beginning to 3.6% by day 12, followed by an increase to 6.5% by the end of storage.
As expected, with prolonged storage and the progressive loss of organic acids, the pH of the fruit juice exhibited an increasing trend (Fig. 6c). The evaluation of pH levels in both control and treated fruits (with free and nano-encapsulated thyme essential oil) revealed statistically significant differences among treatments. The highest pH value (4.25) was recorded in the control fruits after 16 days, while the corresponding values for the E1, E2, NE1, and NE2 treatments were 4.18, 4.23, 4.16, and 4.16, respectively. The most effective preservation of organic acid content and pH stability was achieved in fruits coated with nano-encapsulated thyme essential oil in the chitosan nanogel matrix.
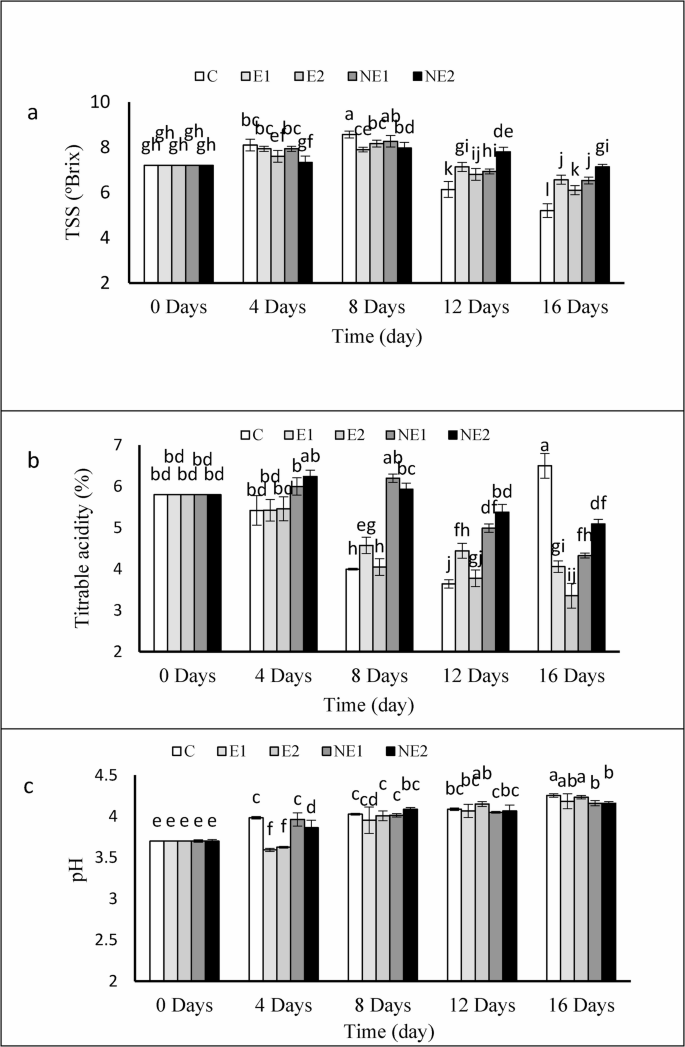
Effects of encapsulated thyme essential oil on TSS a TA b and, pH c of strawberry fruits during 16 days of storage at 4 °C. Data were represented as means ± standard deviation (n = 3). Treatments: C (control), E1 (thyme essential oil coating 600 µL/L), E2 (thyme essential oil coating 1200 µL/L), NE1 (encapsulated thyme essential oil coating 600 µL/L) and NE2 (encapsulated thyme essential oil coating 1200 µL/L)
The reduction in TA and the concurrent increase in pH is commonly associated with enhanced respiratory activity and the progression of fruit senescence. These changes are primarily attributed to the consumption of organic acids during respiration [46]. It appears that the application of essential oil coatings—particularly nano-encapsulated thyme essential oil within a chitosan matrix—effectively reduced metabolic activity and respiration rate, likely by partially blocking stomata and limiting oxygen diffusion. These findings are consistent with previous reports on the use of CMC coatings combined with garlic oil, which extended the shelf life of strawberries by modulating physiological processes [9]. The gradual increase in titratable acidity observed at the end of the storage period in control fruits may be attributed to a potential rise in fungal population. Increased fungal activity can lead to the production of acidic metabolites and mucilage in the fruit tissue—a phenomenon also reported in earlier studies involving edible coatings based on chitosan and Aloe vera extract applied to blueberries [47].
Total anthocyanin content
The changes in total anthocyanin content of strawberry fruits during storage revealed significant differences over time (Fig. 7a). The highest accumulation of anthocyanins was observed in the untreated control fruits and those treated with a high concentration of free essential oil (E2) by the end of the storage period. In contrast, the initial anthocyanin content was 18.34 mg/kg. The lowest increase and the least fluctuation in anthocyanin levels were recorded in fruits treated with nonencapsulated thyme essential oil in chitosan nanogels, followed by treatment E1. The percentage increases in anthocyanin content compared to day 0 for the control, E1, E2, NE1, and NE2 treatments were 55%, 30%, 39%, 31%, and 17.7%, respectively.
The significant rise in anthocyanin, particularly in control fruits, may reflect more advanced ripening during storage and greater weight loss, both of which can influence pigment concentration. However, strawberries treated with thyme essential oil showed lower anthocyanin levels than the untreated fruits. Moreover, statistically significant differences in anthocyanin content were observed among fruits coated with nano-encapsulated thyme essential oil. Anthocyanins, the red pigments in strawberries, are classified as polyphenols. Their biosynthesis is regulated by the activity of phenylalanine ammonia-lyase (PAL) [48]. Key factors influencing total phenolic and anthocyanin synthesis include cultivar and fruit maturity at harvest. The reduced accumulation of anthocyanins in coated fruits could be due to delayed biosynthesis of anthocyanins and other red pigments, consistent with findings from previous studies [49]. Similar results have been reported for other fruits treated with chitosan-based coatings [50]. Notably, more favorable results were achieved when thyme essential oil was nano-encapsulated in chitosan nanogels, effectively mitigating the potential tissue-damaging effects of high concentrations of free essential oil. This encapsulation provided a more controlled release and prolonged effectiveness, as observed in the NE1 and NE2 treatments. These findings align with prior research on the application of lemongrass essential oil in alginate-based edible coatings to extend shelf life and preserve quality in fresh-cut pineapple [51]. The reduced anthocyanin accumulation in treated fruits may also be linked to the semi-permeable properties of thyme essential oil, which can reduce oxygen diffusion and increase localized CO₂ concentration around the fruit surface. This shift in gas composition could suppress biochemical pathways responsible for anthocyanin biosynthesis [52]. Similarly, Pelayo et al. [53] reported that elevated CO₂ levels during storage inhibited anthocyanin synthesis in strawberries. In addition, essential oil treatments can significantly reduce fruit respiration rates, thereby minimizing water loss from the surface, which may explain the lower anthocyanin concentrations in coated fruits compared to uncoated controls. Thus, treatment with essential oils can effectively delay fruit ripening and enhance the postharvest shelf life of strawberries.
Ascorbic acid
One of the key factors in preserving the quality attributes of fruits during storage is their ascorbic acid content [54]. Figure 7b shows the changes in ascorbic acid concentration in strawberries during storage. Total ascorbic acid levels in both control and treated fruits decreased significantly over time. At the end of the storage period, strawberries treated with nano-encapsulated thyme essential oil exhibited higher ascorbic acid levels compared to the control and to those coated with the non-encapsulated essential oil. The most significant reduction in L-ascorbic acid content was observed in the control fruit, decreasing from 52.6 to 17.74 mg/100 g FW, whereas the smallest decrease was seen in treatment NE2, with a final value of 25.98 mg/100 g FW. This decline may be attributed to enhanced oxidation caused by the accumulation of reactive oxygen species, such as superoxide and hydroxyl radicals, in strawberries [14]. The present findings indicate that nanoencapsulation of thyme essential oil in a chitosan-based nanogel can more effectively preserve vitamin C during storage compared to the free essential oil. This may be attributed to the antioxidant properties of the essential oil, as illustrated in Fig. 7b, as well as its ability to reduce oxygen diffusion and consequently lower the respiration rate in treated fruits, thereby slowing the oxidation process of ascorbic acid [55, 56]. These results are consistent with previous studies reporting that edible coatings containing citrus essential oil improved postharvest attributes of strawberries more effectively than lemon oil coatings [43].
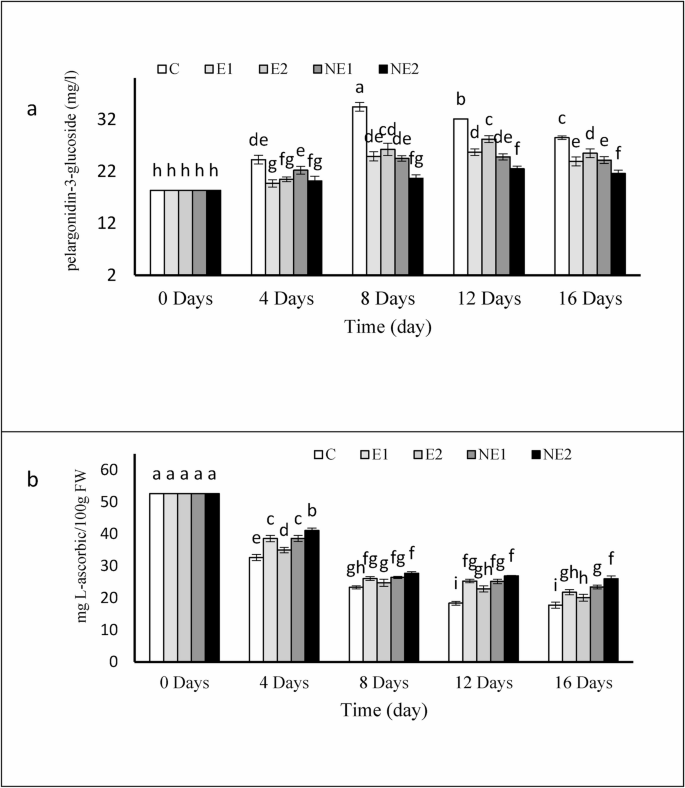
Effects of encapsulated thyme essential oil on the anthocyanin content a and, ascorbic acid b of strawberry fruits during 16 days of storage at 4 °C. Data were represented as means ± standard deviation (n = 3). Treatments: C (control), E1 (thyme essential oil coating 600 µL/L), E2 (thyme essential oil coating 1200 µL/L), NE1 (encapsulated thyme essential oil coating 600 µL/L) and NE2 (encapsulated thyme essential oil coating 1200 µL/L)
Polyphenol oxidase (PPO) activity
During storage, PPO activity in the control fruits increased sharply. In contrast, this increase was significantly (P ≤ 0.05) suppressed in coated samples, particularly in treatments with nanochitosan-encapsulated thyme essential oil (Fig. 8). At the end of the storage period, the lowest PPO activity was observed in NE2 and NE1 treatments, followed by E1. PPO catalyzes the oxidation of phenolic compounds to o-quinones in the presence of oxygen, which are responsible for enzymatic browning in fresh produce tissues. This enzyme can be considered a key indicator for assessing senescence during the postharvest period in fresh fruits and vegetables [57]. The present findings indicate that thyme essential oil, when applied with enhanced stability in the NE2 coating, inhibited oxygen penetration into fruit tissues and prevented tissue degradation, thereby reducing PPO activity. These findings align with previous studies showing that chitosan coatings containing thymol (0.02%) or geraniol (0.04%) effectively reduce PPO activity and extend strawberry shelf life up to 7 days at 4 °C [50]. In the present study, nano-chitosan encapsulation of thyme essential oil appears to further prolong strawberry shelf life compared to these earlier reports.

Effects of encapsulated thyme essential oil on the PPO activity of strawberry fruits during 16 days of storage at 4 °C. Data were represented as means ± standard deviation (n = 3). Treatments: C (control), E1 (thyme essential oil coating 600 µL/L), E2 (thyme essential oil coating 1200 µL/L), NE1 (encapsulated thyme essential oil coating 600 µL/L) and NE2 (encapsulated thyme essential oil coating 1200 µL/L)
Antioxidant enzymes
The activities of the antioxidant enzymes peroxidase (POD) and catalase (CAT) increased markedly in coated strawberries compared with the control fruits throughout the storage period (Fig. 9a and b). In the control group, POD and CAT activities began to decline on days 12 and 8, respectively. In treatments containing free essential oil, the initial increase in enzyme activity was slightly higher than that.
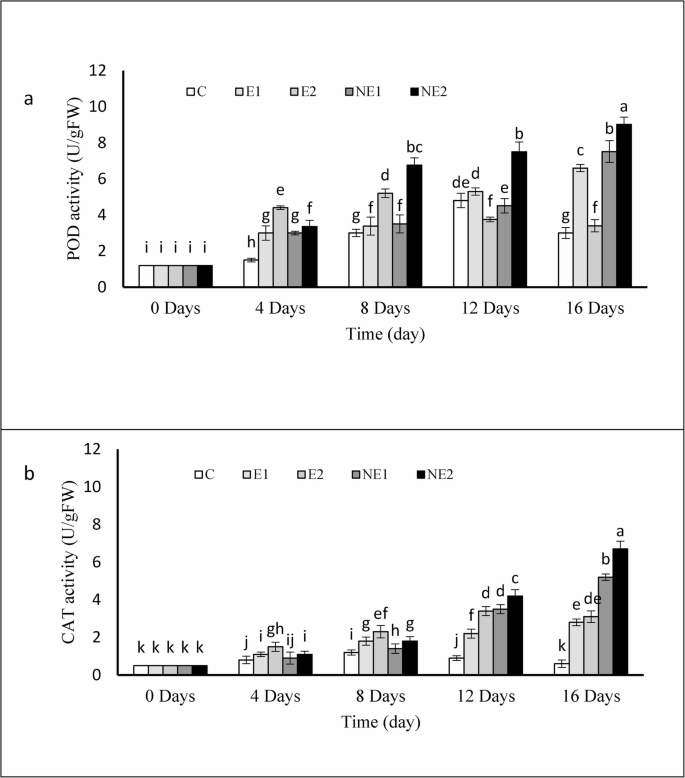
Effects of encapsulated thyme essential oil POD a and, CAT b activity of strawberry fruits during 16 days of storage at 4 °C. Data were represented as means ± standard deviation (n = 3). Treatments: C (control), E1 (thyme essential oil coating 600 µL/L), E2 (thyme essential oil coating 1200 µL/L), NE1 (encapsulated thyme essential oil coating 600 µL/L) and NE2 (encapsulated thyme essential oil coating 1200 µL/L)
in the nanochitosan-encapsulated essential oil treatments until the mid-storage period; however, the latter showed a significantly greater increase thereafter until the end of storage. By the end of storage, POD and CAT activities in the control were recorded at 3.0 and 0.6 U/mg FW, respectively, whereas the highest values were observed in NE2, reaching 9.02 and 6.7 U/mg FW, respectively.
The activation of plant antioxidant defense systems—both enzymatic and non-enzymatic—plays a crucial role in mitigating oxidative damage caused by the accumulation of reactive oxygen species (ROS) [16]. The sustained and pronounced increase in POD and CAT activities observed in strawberries treated with nanochitosan-encapsulated thyme essential oil compared to the control and other treatments clearly indicates suppression of ROS in the treated fruits. Although free thyme essential oil also reduced ROS levels more effectively than the control, its protective effect diminished over time.
POD and CAT mitigate oxidative stress by converting hydrogen peroxide (H₂O₂) into water and oxygen, thereby preventing its deleterious effects [58]. In the present study, the prevention of cellular degradation in strawberries may be attributed to the enhanced POD and CAT activities induced by the encapsulated essential oil, facilitating the breakdown of H₂O₂ into harmless products. In agreement with our findings, Adiletta et al. [59] reported that chitosan/nano-silica coatings increased CAT and SOD activities in loquat fruits, while Hassan et al. [16] demonstrated that thyme essential oil encapsulated in chitosan enhanced CAT and SOD activities in sweet basil leaves, thereby extending their shelf life. It appears that the elevated antioxidant enzyme activities in NE2-treated strawberries contributed to reducing H₂O₂-induced cellular damage and preventing tissue degradation, ultimately prolonging their storage life.
Antioxidant activity
Throughout the storage period, antioxidant activity in all treatments and the control increased until day 8, followed by a gradual decline until the end of storage (Fig. 10). Strawberries coated with chitosan-based formulations exhibited the most minor fluctuations compared with other treatments. In NE2, antioxidant activity increased from 20.1% to 26.16%, whereas the lowest value was recorded in the control fruit at 10.73%. The highest antioxidant activity was observed in coatings containing nanochitosan-encapsulated thyme essential oil at concentrations of 1200 and 600 µL/L (NE2 and NE1), followed by the free essential oil coating (E1).

Effects of encapsulated thyme essential oil on the antioxidant activity of strawberry fruits during 16 days of storage at 4 °C. Data were represented as means ± standard deviation (n = 3). Treatments: C (control), E1 (thyme essential oil coating 600 µL/L), E2 (thyme essential oil coating 1200 µL/L), NE1 (encapsulated thyme essential oil coating 600 µL/L) and NE2 (encapsulated thyme essential oil coating 1200 µL/L)
The decline in antioxidant activity in the control fruit at the end of storage can be attributed to natural senescence and spoilage. These results suggest that encapsulation of thyme essential oil in nanochitosan not only extends shelf life but also preserves higher antioxidant activity in strawberries over prolonged storage. Thymol, one of the most common phenolic compounds in essential oils, has been previously reported to possess potent antioxidant properties [50, 60]. Consistent with our findings, Wang and Gao [29] reported that chitosan coatings enhanced radical scavenging capacity, phenylpropanoid compound levels, and antioxidant enzyme activities in strawberries. Moreover, the antioxidant activity of chitosan can be further enhanced by incorporating compounds such as certain sugars, essential oils, and gelatin [61]. Our findings are also in agreement with earlier reports indicating that the antioxidant mechanism of chitosan may arise from its ability to chelate metal ions such as copper and iron at enzyme active sites, thereby inactivating oxidizing enzymes, and/or to interact with lipids in these enzymes [50, 60].
Pathogenic contamination assessment
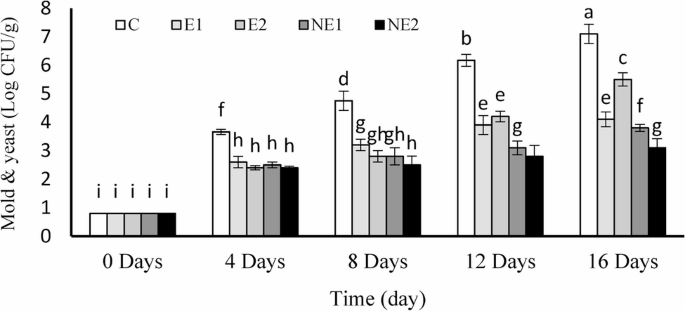
Microbial colony enumeration is an effective method to evaluate the antimicrobial performance of different coating formulations for strawberry preservation. According to established standards, fresh fruits or vegetables are considered unsuitable for consumption when total microbial counts exceed 4 Log CFU/g [62]. As shown in Fig. 11, a gradual increase in microbial colony numbers over time was evident across all treatments.
Effects of encapsulated thyme essential oil on the decay incidence of strawberry fruits during 16 days of storage at 4 °C. Data were represented as means ± the standard deviation (n = 3). Treatments: C (control), E1 (thyme essential oil coating 600 µL/L), E2 (thyme essential oil coating 1200 µL/L), NE1 (encapsulated thyme essential oil coating 600 µL/L) and NE2 (encapsulated thyme essential oil coating 1200 µL/L)
Among the groups, untreated fruits (control) exhibited the highest microbial proliferation. At the beginning of storage, microbial counts in the control group were 0.85 Log CFU/g, which rose to 4.75 Log CFU/g by day 8—exceeding the permissible consumption threshold. By day 16, colony counts in the control group were 1.5-fold higher than those on day 8, indicating severe microbial contamination. In contrast, samples treated with NE2 showed a markedly slower increase in colony numbers. However, counts rose during storage, the final value was only two logarithmic cycles higher than the initial level and remained below the unfit-for-consumption limit. This suggests that NE2 treatment significantly suppressed microbial growth up to day 16. Under cold storage conditions, the shelf life of strawberries varied between 4 and 16 days depending on the treatment. The application of free thyme essential oil in coatings also delayed microbial growth, extending fruit longevity by approximately four and eight days in treatments E1 and E2, respectively.
Since fungal spoilage is one of the primary factors reducing strawberry quality and shelf life, a separate evaluation of yeast and mold growth across treatments was conducted [63]. As depicted in Fig. 11, yeast and mold counts increased progressively in all groups during storage. However, coatings containing chitosan-encapsulated thyme essential oil exhibited slower fungal proliferation, particularly in NE2, where the essential oil concentration was doubled. In this treatment, yeast and mold counts were reduced from 7.41 to 3.10 Log CFU/g compared with the control, representing a decrease of more than two logarithmic cycles. These findings are consistent with the results of De Oliveira Filho et al. [64] and Li et al. [44], who also reported that nano-chitosan coatings containing thyme essential oil and nano-emulsions with Cymbopogon martinii or Mentha spicata essential oils significantly inhibited yeast and mold growth compared to essential-oil-free controls [44, 64]. This antimicrobial effect is likely due to the combined action of chitosan and thyme essential oil, which disrupts fungal membrane integrity, thereby reducing contamination and inhibiting respiration [65].
Overall, these results indicate that nanochitosan-encapsulated thyme essential oil at a concentration of 1200 µL/L plays a critical role in reducing fungal growth and extending strawberry shelf life. The NE2 coating formulation effectively prevented yeast and mold proliferation, thereby mitigating fruit spoilage during storage.