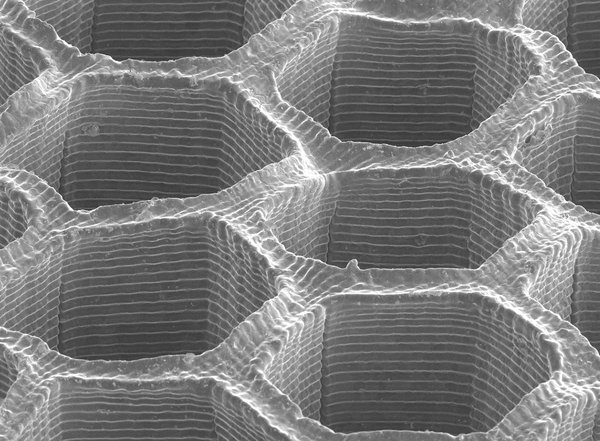
Caltech scientists have developed a method to create metallic objects of a precisely specified shape and composition, giving them unprecedented control of the metallic mixtures, or alloys, they create and the enhanced properties those creations will display. Want a stent that is biocompatible and mechanically robust? How about strong but lightweight satellite components that can operate in space for decades? The new technique can tell scientists exactly which combination of metals will yield the best product. In addition, it offers a route to making alloys with beneficial properties determined by their underlying structure, such as surprisingly strong copper–nickel alloys.
“If you look at how metallurgy has been done for centuries, in broad strokes, you nearly always start with a raw ore, which is then thermally and/or chemically treated and refined, to produce the desired metal or alloy. And basically, the mechanical properties of the metals produced this way are limited,” says Julia R. Greer, the Ruben F. and Donna Mettler Professor of Materials Science, Mechanics and Medical Engineering at Caltech and executive officer for applied physics and materials science at Caltech. “What we are showing is that you can actually fine-tune the chemical composition and the microstructure of metallic materials, substantially enhancing their mechanical resilience.”
Greer and her colleagues describe the new method in a paper published online by the journal Small. The lead author of the paper is Thomas T. Tran (PhD ’25), and the second author is Rebecca Gallivan (PhD ’23), a former member of the Greer lab who is now an assistant professor of engineering at Dartmouth College.
The new technique builds on previous work from the Greer lab in which the scientists showed how to use a form of 3D printing, or additive manufacturing, to make complex microscale metal structures. Previously, the technique, called hydrogel-infusion additive manufacturing (HIAM), had been used to carefully build structures from a single type of metal. In the new work, Tran has figured out a way to infuse more than one metal at a time, creating copper–nickel alloys containing custom percentages of copper and nickel—differences that matter when it comes to material properties.
The process begins with 3D printing of an organic hydrogel material, depositing the polymer resin precisely where it is wanted, layer by layer, to create a gel-like scaffold. That scaffold is then infused with metal ions by pouring a liquid solution of metallic salts over the structure. Next, in a process called calcination, the scientists burn the material, removing all the organic content and leaving behind the metals. Since this is done in the presence of oxygen, what is left is a mixture of metal oxides.
In an innovative next step, called reductive annealing, Tran raises the temperature in a hydrogen environment causing most of the oxygen to diffuse back out of the solid; it then reacts with hydrogen to form water vapor. This leaves behind a metallic structure of the desired shape that is an alloy of the two added metals.
“The composition can be varied in whatever manner you like, which has not been possible in traditional metallurgy processes,” Greer explains. “One of our colleagues described this work as bringing metallurgy into the 21st century.”
By analyzing the microstructure, which includes the orientation of the individual crystal grains and the boundaries among them within the alloys they produced, and by mechanically testing the materials, the scientists were able to reveal more about the special alloys made with the new technique.
“This lays the groundwork for thinking about 3D-printed alloy design in a unique way from other microscale additive manufacturing techniques,” says Gallivan. “We see that the processing environment leads to very different microstructures in comparison to other methods.”
Using a transmission electron microscope (TEM) at the UC Irvine Materials Research Institute, the Caltech researchers were able to show that alloys produced using their HIAM method form more homogenously, resulting in higher degrees of symmetry throughout their crystal structure, Tran explains. The shape, size, and orientation of metal grains are influenced by the transition between oxide and metal during reductive annealing. At elevated temperatures, pores form as water vapor escapes. Metal grain growth is slowed by these pores and oxides. The new work shows that this growth is modified by the types of oxides present in these 3D-printed metals.
As a result, the new paper shows that the strength of alloys created by HIAM is determined not only by the size of the grains within the metals—as was previously thought— but also by their composition. A Cu12Ni88 alloy with 12 atoms of copper for every 88 atoms of nickel, for example, is nearly four times as strong as a Cu59Ni41 alloy that has copper and nickel in a 59/41 ratio.
The TEM studies also revealed that the HIAM process leaves these alloys with tiny oxide inclusions that contribute to the materials’ exceptional strength. “Because of the complex ways in which metal is formed during this process, we find nanoscale structures rich with metal–oxide interfaces that contribute to the hardening of our alloys by up to a factor of four,” Tran says.
The paper is titled “Multiscale Microstructural and Mechanical Characterization of Cu–Ni Binary Alloys Reduced During Hydrogel Infusion-Based Additive Manufacturing (HIAM).” The work was supported by the US Department of Energy’s Basic Energy Sciences program and by a National Science Foundation graduate fellowship.