Introduction: Traditional Chinese Medicine (TCM) provides a practical and safe approach to smoking cessation. However, research examining its integration into community-based smoking cessation programs in Chinese mainland remains limited.
Methods: This cluster randomized controlled trial selected 20 matched communities in Qingdao and randomly assigned them in a 1∶1 ratio to intervention or control groups, with 10 communities per group. Community health centers recruited voluntary smokers seeking cessation as study participants. The final sample comprised 239 participants in the intervention group and 250 in the control group, totaling 489 participants. The intervention group received a comprehensive TCM-based community intervention incorporating acupuncture and auricular acupressure, while the control group received standard self-help smoking cessation materials. Follow-up assessments were conducted at one, three, and six months post-enrollment. Logistic regression models were employed to evaluate the intervention’s impact on smoking cessation outcomes.
Results: Logistic regression analysis adjusted for covariates demonstrated that the intervention group achieved significantly superior smoking cessation outcomes at all follow-up time points compared to the control group. At 6 months, participants in the intervention group showed significantly higher probabilities of achieving sustained cessation [adjusted odds ratio (aOR)=2.44, 95% confidence interval (CI): 1.08, 5.50], attempting cessation (aOR=5.01, 95% CI: 3.14, 7.99), reducing smoking consumption (aOR=2.99, 95% CI: 2.00, 4.45), and maintaining 7-day point prevalence abstinence (aOR=3.76, 95% CI: 2.04, 6.90).
Conclusions: These findings provide compelling evidence supporting the integration of TCM smoking cessation therapies into community-based cessation services. The results offer innovative perspectives and empirical evidence for advancing smoking intervention models in public health practice.
Smoking cessation represents the most effective strategy for reducing population-level smoking prevalence. However, unassisted attempts to quit achieve success rates of only 3.00%–5.00% (1). While evidence-based research in modern medicine has established Traditional Chinese Medicine (TCM) acupuncture as a feasible, effective, and low-risk therapeutic approach for smoking cessation (2), a significant research gap persists regarding the effectiveness of community-based acupuncture interventions for smoking cessation. This community-based trial conducted in Qingdao, China, from December 2023 to December 2024, evaluated the effectiveness of an integrated TCM smoking cessation intervention. The study enrolled 489 eligible participants, with 239 assigned to the TCM intervention group and 250 to the control group. At each follow-up assessment, the intervention group demonstrated significantly higher rates of continuous abstinence, seven-day point prevalence of abstinence, smoking reduction, and quit attempts compared to the control group. Adjusted logistic regression analysis revealed that relative to controls, the intervention group had 2.44 times higher odds of achieving continuous abstinence [95% confidence interval (CI): 1.08, 5.50], 5.01 times greater likelihood of attempting smoking cessation (95% CI: 3.14, 7.99), 2.99 times increased probability of smoking reduction (95% CI: 2.00, 4.45), and 3.76 times elevated odds of seven-day abstinence (95% CI: 2.04, 6.90) at six-month follow-up.
Based on the sample size formula for cluster randomized controlled trials, each group required a minimum of 72 participants. To ensure adequate statistical power, we recruited additional participants beyond this threshold. We selected 20 matched communities in Qingdao and randomly assigned them in a 1∶1 ratio to either intervention or control groups. Community health service centers recruited approximately 25 participants per community from voluntary quitters who had at least one year of smoking history and had smoked daily in the previous month. The control group received standard self-help smoking cessation materials distributed by community health workers (Figure 1). Given the distinctive nature of TCM treatment protocols, blinding participants to group assignment was not feasible. The intervention group participated in a comprehensive TCM smoking cessation program comprising two key components: TCM therapeutic services in the form of body acupuncture administered 2 to 3 times weekly for eight weeks, combined with auricular acupressure involving seed replacement every 2 to 3 days for eight weeks. Trained community physicians performed all acupuncture and auricular point pressing procedures following standardized protocols developed by the China Academy of Chinese Medical Sciences (CACMS). To ensure treatment consistency across all sites, these physicians completed two intensive training sessions provided by CACMS, focusing on precise point location, needling techniques, and auricular pressing methods (3). Supportive environmental interventions encompass comprehensive public education on tobacco-related health risks, disseminated through multiple channels, including timed releases of risk information via community WeChat groups and offline materials such as posters and bulletin boards. The program featured six educational lectures at community health centers covering topics including smoking health risks, cessation benefits, and firsthand quit-smoking experiences, with each lecture reaching at least 50 community residents. Additional activities included smoking cessation competitions, smoke-free family initiatives, smoke-free community programs, and complimentary TCM medical consultations after each lecture. Community workers implemented supportive activities to create an environment conducive to smoking cessation.
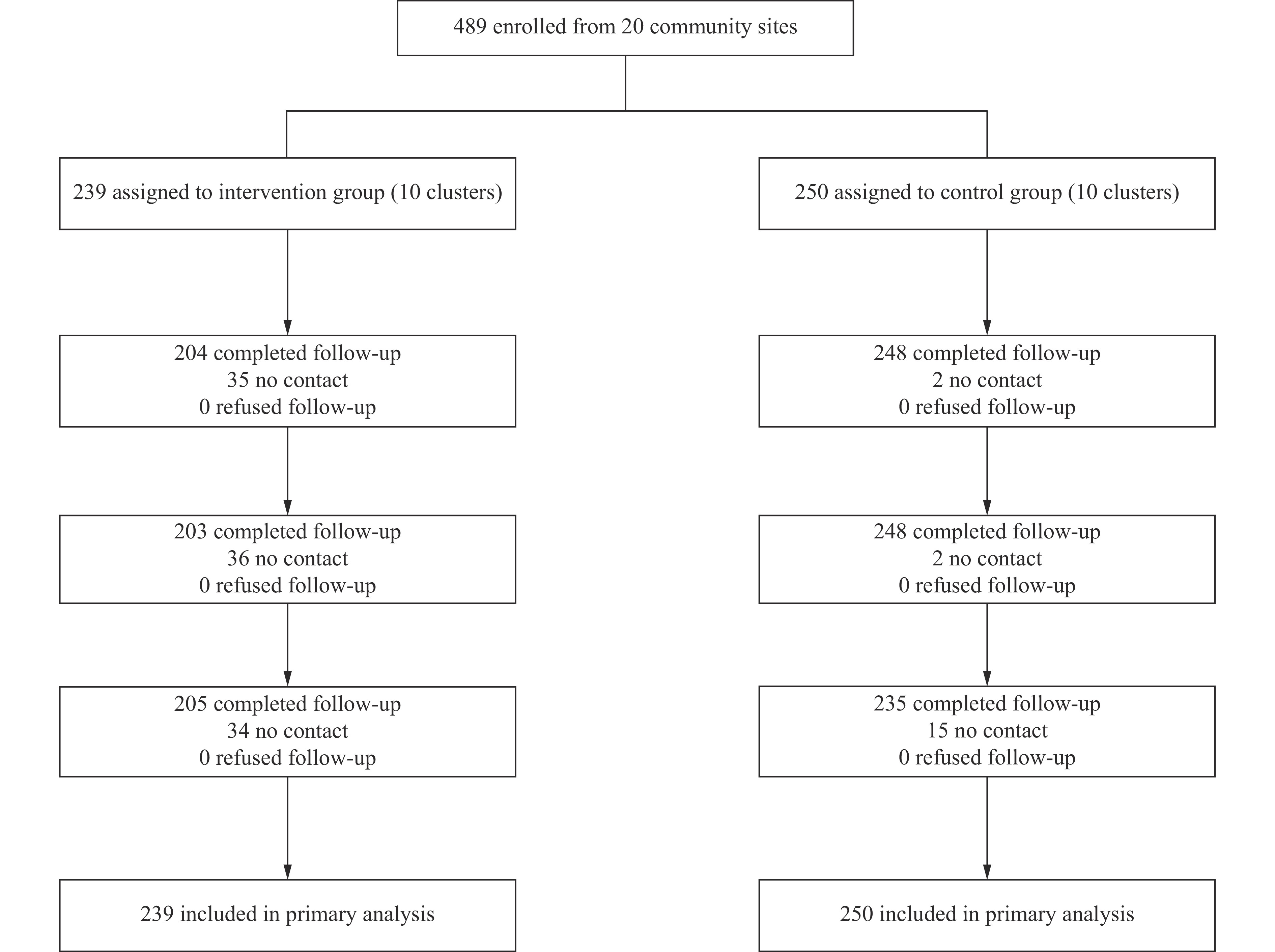
Flowchart of participant recruitment and progression throughout the study — Qingdao City, China, 2023.
Participants underwent follow-up assessments at one, three, and six months post-intervention. The primary outcome was continuous abstinence rate (CAR), while secondary outcomes included seven-day point prevalence of abstinence rate (PPAR), smoking reduction rate, and quit attempt rate at each follow-up timepoint. We applied the following standardized definitions: a quit attempt was defined as self-reported abstinence lasting ≥24 hours; seven-day PPAR required self-reported continuous abstinence for ≥7 days preceding the follow-up assessment; CAR indicated sustained self-reported abstinence maintained since enrollment; and smoking reduction was defined as a ≥50% decrease in daily cigarette consumption compared to baseline levels (excluding participants who reported complete abstinence). All analyses adhered to intention-to-treat (ITT) principles, with participants lost to follow-up conservatively classified as current smokers. Participants who missed all three follow-up assessments were considered lost to follow-up while remaining included in ITT analysis.
Data analysis was performed using SPSS (version 25, IBM Corporation, Armonk, US) and R software (version 4.4.3, R Foundation for Statistical Computing, Vienna, Austria). Continuous variables following normal distributions were presented as mean ± standard deviation (SD), while non-normally distributed variables were summarized as median (interquartile range, IQR). Categorical variables were described using frequencies and percentages. We conducted logistic regression analysis to identify factors associated with six-month smoking cessation outcomes, with results reported as odds ratio (OR) and 95% CI. All statistical tests were two-tailed, with P<0.05 considered statistically significant.
This study enrolled 489 smokers, with participants distributed by 239 in the intervention group and 250 in the control group. The study population was predominantly male (98.57%) with a mean age of 47.75 years. The majority of participants were married (87.53%) and employed (66.05%), while 40.69% had achieved college-level education or higher. Participants consumed an average of 14.13±8.12 cigarettes per day at baseline (Table 1).
Table 1.
Baseline characteristics of participants — Qingdao City, China, December 2023 – December 2024 [N (%)].
At each follow-up time point, the intervention group consistently demonstrated significantly superior rates across all smoking cessation outcomes compared to the control group. Specifically, the intervention group achieved higher quit attempt rates, seven-day PPAR, CAR, and smoking reduction rates at 1-month (43.51% vs. 14.00%, 26.36% vs. 8.80%, 23.85% vs. 7.60%, and 37.24% vs. 22.40%; P<0.05), 3-month (54.81% vs. 19.20%, 29.71% vs. 6.80%, 19.25% vs. 4.40%, and 43.10% vs. 30.40%; P<0.05), and 6-month follow-ups (61.09% vs. 22.40%, 30.96% vs. 8.00%, 17.57% vs. 4.00%, and 46.03% vs. 22.80%; P<0.05).
Logistic regression analysis was performed to identify predictors of six-month CAR, incorporating all aforementioned variables. The results revealed that participants in the intervention group demonstrated 2.44 times greater likelihood of achieving sustained smoking cessation at six months compared to the control group [adjusted OR (aOR)=2.44, 95% CI: 1.08, 5.50]. Additionally, higher educational attainment (aOR=2.37, 95% CI: 1.44, 3.89) and poorer perceived health status (aOR=2.16, 95% CI: 1.03, 4.54) significantly enhanced the probability of successful cessation. Conversely, higher daily cigarette consumption substantially reduced cessation success (aOR=0.93, 95% CI: 0.89, 0.97). The comprehensive results are presented in Table 2.
Table 2.
Logistic regression analysis of influencing factors of the six-month CAR — Qingdao City, China, December 2023 – December 2024 (n=489).
Identical logistic regression models were employed to examine intervention effects on six-month quit attempt rates, seven-day PPAR, and smoking reduction rates. The analysis demonstrated that participants in the intervention group exhibited 5.01 times higher odds of making quit attempts (aOR=5.01, 95% CI: 3.14, 7.99), 2.99 times greater odds of achieving smoking reduction (aOR=2.99, 95% CI: 2.00, 4.45), and 3.76 times elevated odds of attaining seven-day PPAR (aOR=3.76, 95% CI: 2.04, 6.90) compared to the control group, with all differences reaching statistical significance (Table 3).
Table 3.
Comparison of cessation outcomes between the intervention and control groups at a six-month follow-up — Qingdao City, China, December 2023 – December 2024.
To address potential confounding from baseline differences, we conducted comprehensive sensitivity analyses. The results confirmed that the intervention’s beneficial effects on all outcome variables maintained statistical significance (P<0.05) across all model specifications, with consistent positive effect directions (β>0), demonstrating the robustness of our primary findings (Table 4).
| Elements | Model 1 (unadjusted) | Model 2 (adjusted)* | Model 3 (adjusted)† | |||||
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |||
| Quit attempt rate | 5.44 (3.66, 8.07) | <0.01 | 4.27 (2.73, 6.68) | <0.01 | 5.01 (3.14, 7.99) | <0.01 | ||
| Smoking reduction rate | 2.89 (1.96, 4.26) | <0.01 | 2.89 (1.96, 4.26) | <0.01 | 2.99 (2.00, 4.45) | <0.01 | ||
| Seven-day PPAR | 5.16 (3.03, 8.79) | <0.01 | 3.43 (1.88, 6.26) | <0.01 | 3.76 (2.04, 6.90) | <0.01 | ||
| CAR | 5.12 (2.50, 10.46) | <0.01 | 2.49 (1.13, 5.48) | 0.02 | 2.44 (1.08, 5.50) | 0.03 | ||
| Abbreviation: CI=confidence interval; PPAR=point prevalence of abstinence rate; CAR=continuous abstinence rate. * Covariates in model 2 included age, marital status, education, health status, chronic non-communicable diseases, Fagerström test for nicotine dependence. † Covariates in model 3 included all baseline characteristics. |
||||||||
Table 4.
Sensitivity analysis of the impact of intervention effects on various outcome variables at the 6-month follow-up.