President Cyril Ramaphosa has this morning, 12 January 2026, arrived in the United Arab Emirates (UAE) at the invitation of His Highness Sheikh Mohammed bin Zayed Al Nahyan, President of the UAE, to participate in the Abu Dhabi Sustainability…
Author: admin
-
Balochistan: 14-year | DD News On Air
A 14-year-old boy was reportedly shot dead in Balochistan, while six Baloch men have disappeared in separate incidents. According to the Baloch Yakjehti Committee (BYC), 14-year-old Raahi Assa was killed in the main bazaar of Hoshap, Kech….
Continue Reading
-

Economic-clinical burdens of tuberculosis treatment in vulnerable pati
Introduction
Tuberculosis (TB) is an infectious disease caused by a mycobacterium, Mycobacterium tuberculosis (known as Koch’s bacillus), which most often affects the lungs (pulmonary tuberculosis) but can also affect other organs…
Continue Reading
-
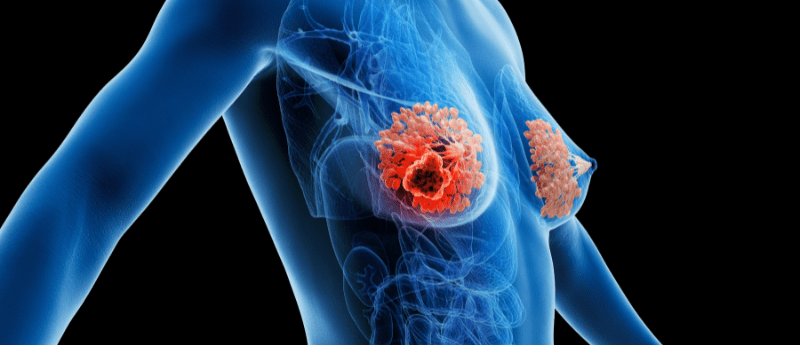
Cholesterol-lowering drug found to directly overcome chemotherapy resistance in TNBC cells
Original story from Korea University College of Medicine
Researchers discover that cholesterol-lowering drug, called pitavastatin, can overcome chemotherapy resistance in triple-negative breast cancer (TNBC) cells.
A Korea University…
Continue Reading
-

Roadside bomb targeting police vehicle kills 6 officers in northwest Pakistan
DERA ISMAIL KHAN, Pakistan — A roadside bomb targeting a police vehicle killed six officers in northwestern Pakistan near the Afghan border on Monday, police said, underscoring a surge in militant violence in the region.
The blast struck in Tank…
Continue Reading
-
Amna Baloch stresses cyber security’s role in national resilience – RADIO PAKISTAN
- Amna Baloch stresses cyber security’s role in national resilience RADIO PAKISTAN
- FS Amna Baloch highlights growing significance of cybersecurity for national resilience Associated Press of Pakistan
- PKCERT, Kaspersky sign MoU to strengthen…
Continue Reading
-

This Is What the Year of the Fire Horse Means for You
Whether you follow the Chinese Zodiac or not, if you’re in any way online then you’re probably aware that 2026 is the Year of the Horse. From TikTok videos promoting the general embrace of “horse energy” (different from horse girl energy,…
Continue Reading
-
Chinese medicines emerge as a bridge of health amid Afghanistan’s healthcare struggles-Xinhua
KABUL, Jan. 12 (Xinhua) — Esmatullah, a 32-year-old taxi driver in Kabul and the sole breadwinner for a family of 10, spends more than 1,000 afghanis (nearly 16 U.S. dollars) per month on medicines prescribed for his wife, who suffers from a…
Continue Reading
-

Reading Prison plans set to be outlined in the summer
The foundation is in the process of appointing a new architect in the UK after previously employing one based in Italy.
The latter completed several designs which were sent to Reading Council, the community and Historic England for approval.
But…
Continue Reading
-

Medical school dean wants more local doctors
The school opened in 2021 after several delays and concerns over sustainable funding.
It was hoped the school’s opening in the city would eventually help to address a relative shortage of doctors in Northern Ireland.
Bazira said recruiting local…
Continue Reading