Author: admin
-
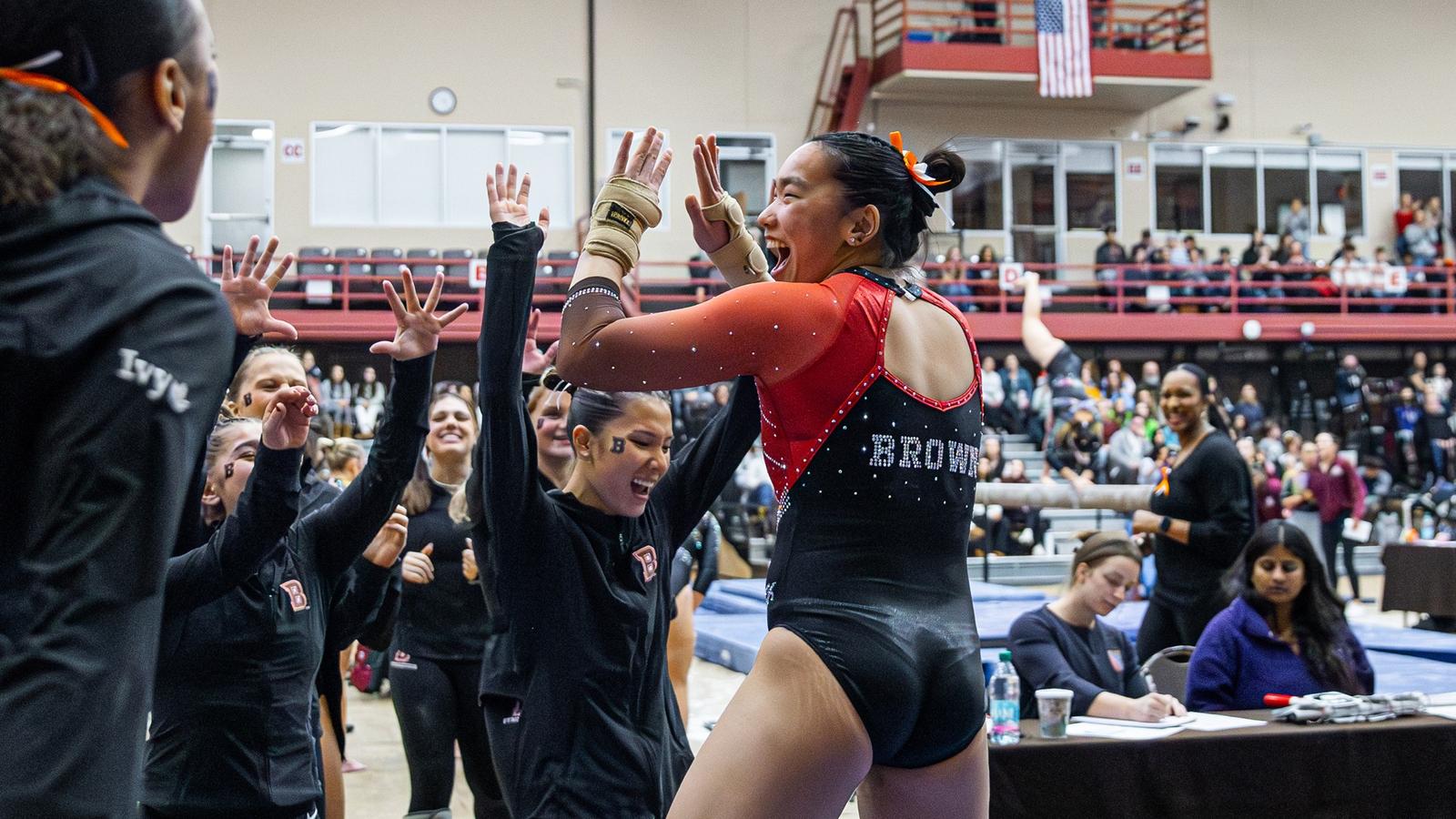
Gymnastics Posts Season-Opening Program Record Score in Sunday Quad Meet
PROVIDENCE, R.I. – The Brown gymnastics team opened its 2026 season on Sunday afternoon by posting a score of 193.025, the highest score in a season opening meet in program history. Opening the season at home for the first time since 2017,… -

Giles’ Career-High 33 Propels Wichita State to Win over UNT

Esperion Provides Business Update at 44th Annual J.P. Morgan Healthcare Conference
– Reports $156 to $160 Million in Preliminary* Full-Year 2025 U.S. Net Product Sales, a 35% to 38% Increase Compared With Full-Year 2024 –
–Total Preliminary* Revenue of $400 to $408 million, a 20% to 23% Increase Compared With Full-Year 2024, and ~55% to 59% Increase Excluding One-Time Milestones –
– Cash and Cash Equivalents of Approximately $168 Million* at Year-End 2025 –
– Q4 Retail Prescription Equivalents Grew 34% Y/Y and 11.3% Q/Q –
– Expects Operating Expenses of Between $210 Million and $245 Million for Full-Year 2026 –– Introduces Vision 2040 Growth Strategy Focused on Global Growth of Cardiometabolic Franchise and Expansion of Pipeline into Rare Hepatic and Renal Diseases –
ANN ARBOR, Mich., Jan. 11, 2026 (GLOBE NEWSWIRE) — Esperion (NASDAQ: ESPR) today provides preliminary financial results for the full-year 2025, including U.S. net product sales, cash and cash equivalents and expectations for 2026 operating expenses.
“2025 marked the most successful year in Esperion’s history, driven by exceptional execution across our U.S. commercial strategy, expanded global reach and performance, and significant progress across our pipeline – all while delivering meaningful growth in our cardiovascular franchise and strengthening our financial position. These achievements set the stage for something far bigger: Vision 2040. This bold roadmap reflects our commitment to transform Esperion into a multi-product, sustainable, innovation-driven global pharmaceutical company that not only leads in cardiovascular disease prevention but also addresses a broader spectrum of unmet medical needs,” said Sheldon Koenig, Chief Executive Officer of Esperion.
“By 2040, we envision Esperion as a company with multiple blockbuster products on the market, a robust pipeline of next-generation therapies, and a proven commercial infrastructure that makes us a partner of choice. Our deep expertise in ACLY biology and our relentless focus on patient impact will fuel this evolution. Vision 2040 is more than a strategy—it’s a promise to patients, providers, and shareholders that Esperion will continue to lead with science, scale with purpose, and deliver enduring value for decades to come,” concluded Mr. Koenig.
Introducing Vision 2040
Esperion is pleased to introduce its Vision 2040 that outlines its long-term strategy to evolve into a sustainable, innovation-driven, global pharmaceutical company that is anchored by leadership in cardiometabolic indications including rare and orphan diseases. Esperion’s goal is to leverage the billion-dollar opportunity in its current cardiovascular disease prevention franchise to build a global pharmaceutical company with a growing product portfolio of at least five marketed products and a dynamic discovery engine producing a robust pipeline that addresses a variety of diseases of unmet medical need.
A central component of Vision 2040 is to expand our product portfolio and advance our next-generation development pipeline. The company plans to leverage its proven commercial infrastructure to become a partner-of-choice through acquisition, in-licensing, co-promotion, and revenue-share opportunities. At the same time, Esperion will build on its deep domain expertise in ACLY biology to diversify our therapeutic focus and advance a series of novel product candidates, each with the power to change lives and the potential to become blockbuster products.
Together, these pillars form the foundation for Vision 2040 – a roadmap designed to transform Esperion by uniting scientific leadership, strategic business development, and operational execution. Esperion is positioned to expand its reach, accelerate innovation, and deliver lasting benefits to patients, partners and shareholders.
Advancing the U.S. Commercial Strategy
NEXLETOL® (bempedoic acid) and NEXLIZET® (bempedoic acid and ezetimibe) are approved by the U.S. Food and Drug Administration to help prevent heart attacks and cardiovascular procedures in both primary and secondary prevention patients, regardless of statin use. The treatable population represents more than 70 million patients in the U.S. alone. Esperion is currently focusing its commercialization efforts on the statin intolerant or statin resistant market, which represents approximately 30% of the overall market. To address this key market segment, Esperion has ramped up its sales efforts, developed a powerful suite of new promotional materials, created a bold new consumer campaign, enhanced its patient support programs, and continued working with payers to ensure broad patient access.
Importantly, Esperion is undertaking a series of strategic initiatives aimed at reinforcing the long-term protection of the bempedoic acid franchise, including potential extensions of market exclusivity and the introduction of a triple combination product that could provide a level of efficacy with the potential to rival existing injectable and emerging oral therapies.
- Achieved $156 to $160 million in preliminary* full-year 2025 U.S. net product sales, representing a 35% to 38% increase compared with full-year 2024
- Q4 retail prescription equivalents showed 34% Y/Y and 11.3% sequential Q/Q growth.
- Reached settlement agreements with four key ANDA filers, including Dr. Reddy’s Laboratories, restricting generic entry by these parties until April 2040 and leaving no remaining challenges regarding the validity or infringement of U.S Patent No. 7,335,799 in the pending patent litigation. Certain of Esperion’s patents that remain subject to the pending patent litigation are scheduled to expire in March 2036, while others are scheduled to expire in June 2040.
- Significantly strengthened access and reimbursement support for NEXLETOL and NEXLIZET, now exceeding 90% of commercial lives and 90% of Medicare beneficiaries covered, with all national commercial and Medicare payers covering all indications.
- Introduced the “Can’t take a statin? Make NEXLIZET happen!” campaign, which has already increased brand awareness and improved healthcare providers’ perception of NEXLETOL and NEXLIZET among statin-intolerant or resistant patients.
- Enabled the expansion of healthcare providers prescribing NEXLETOL and NEXLIZET from 36,311 to 44,991, a 24% increase in 2025 with strengthened reimbursement and award-winning marketing and educational initiatives.
- Expect bempedoic acid products to be included in the upcoming U.S. Dyslipidemia Guidelines in Q1 2026, which would be a major catalyst to drive adoption and growth of the bempedoic franchise.
- To leverage the strengthened reimbursement, potential expanded timeline to generic entry, and anticipated U.S. guideline inclusion, the Company plans to expand its U.S. commercial efforts through enhanced sales and marketing investments.
- Plans to expand its revenue opportunity in the cardiovascular prevention market with the introduction of two triple combination products. The published literature suggests that the triple combination products can lower LDL-C in excess of 60%. This level of efficacy has the potential to rival existing injectable and emerging oral therapies, offering a valuable oral option for both patients and physicians. The company expanded its partnerships with regulatory experts and others in the field to advance this important work with a goal to complete the clinical requirements and commercialize triple combinations in 2027.
Global Expansion
Cardiovascular (CV) disease remains the leading cause of death worldwide, and Esperion continues to make meaningful progress delivering its bempedoic acids products to patients who are unable to achieve their low-density lipoprotein cholesterol (LDL-C) goals and remain at heightened risk of CV disease or a CV event, such as a heart attack. Together with our global partners, we have made significant advancements expanding access to bempedoic acid internationally, strengthening the global footprint of the franchise.
- Daiichi Sankyo Europe, Esperion’s strategic partner across Europe, continued to deliver double digit quarterly growth across key EU markets, and expanded availability of bempedoic acid therapies across 30 countries in the European Union, with more than 600,000 patients treated to date.
- Bempedoic acid was included as a Class I, Level A recommendation in the 2025 ESC/EAS guidelines.
- Secured regulatory and reimbursement approval for NILEMDO in France, one of the largest markets in Europe.
- Announced the development of oral triple combination lipid-lowering tablets, with SANTORINI simulations showing improved LDL-C goal attainment aligned with the 2025 ESC/EAS guidelines.
- Otsuka Pharmaceutical Co., Ltd., Esperion’s strategic partner in Japan, received regulatory approval and favorable National Health Insurance price listing, which resulted in a $90 million total payment to Esperion.
- Successful commercial launch in late 2025 sets the stage for meaningful growth in 2026.
- Japan is the third largest global market for cardiovascular prevention, representing significant long-term growth opportunity for NEXLETOL.
- HLS Therapeutics, Esperion’s strategic partner in Canada, received regulatory approval for NILEMDO in late 2025, with approval for NEXLIZET expected in 2026.
- Esperion continues to expect its partner in Israel, Neopharm Israel, to receive regulatory approval to market NEXLETOL and NEXLIZET in the first half of 2026.
- CSL Seqirus, the Company’s partner in Australia and New Zealand, filed a marketing application in Australia for NEXLETOL and NEXLIZET in July 2025, and expects market approval in Q4 2026.
R&D Pipeline
Esperion plans to advance its promising ACLY-focused pipeline, leveraging its established leadership in ACLY biology to pursue new therapeutic opportunities and develop next-generation inhibitors designed to address multiple life-threatening diseases. ACLY is a critical metabolic enzyme positioned at the intersection of nutrient catabolism and cholesterol and fatty acid biosynthesis, making it an attractive target for broad therapeutic intervention.
- Nominated ESP-2001, a highly specific allosteric ATP-citrate lyase inhibitor, as preclinical development candidate for the treatment of primary sclerosing cholangitis (PSC).
- Initiated Investigational New Drug-enabling studies for ESP-2001, with the goal of submitting an IND to the U.S. Food and Drug Administration (FDA) to begin first-in-human clinical studies in 2026.
- With an estimated prevalence of approximately 76,000 diagnosed PSC patients across the U.S. and Europe, and with no approved treatment options, ESP-2001 – a wholly owned asset for which Esperion retains exclusive global development and commercialization rights – represents a potential blockbuster market opportunity of more than $1 billion annually.
- ESP-2001 has potential eligibility for Orphan Drug and Fast Track designations from the U.S. FDA, as well as PRIME designation from the European Medicines Agency.
Financials
Esperion completed a $75.0 million capital raise in 2025, enhancing financial flexibility to support continued commercial expansion and pipeline development.
Esperion introduces its expectations for full-year 2026 operating expenses to be in the range of $210 million to $245 million, including $15 million in non-cash expenses related to stock compensation.
J.P. Morgan Healthcare Conference Presentation
Esperion will present at the 44th Annual J.P. Morgan Healthcare Conference on Wednesday, January 14, 2025, at 2:15 p.m. PT (5:15 p.m. ET).
The live webcast can be accessed on the investor and media section of the Esperion website. Access to the webcast replay will be available approximately two hours after the completion of the call and will be archived on the Company’s website for approximately 90 days.
* The preliminary selected financial results are unaudited, subject to adjustment, and provided as an approximation in advance of the Company’s announcement of complete financial results in March 2026.
INDICATION
NEXLIZET and NEXLETOL are indicated:- The bempedoic acid component of NEXLIZET and NEXLETOL is indicated to reduce the risk of major adverse cardiovascular events (cardiovascular death, myocardial infarction, stroke, or coronary revascularization) in adults at increased risk for these events who are unable to take recommended statin therapy (including those not taking a statin).
- NEXLIZET, to reduce LDL-C in adults with hypercholesterolemia, including HeFH.
- NEXLETOL, in combination with other LDL-C lowering therapies, or alone when concomitant LDL-C lowering therapy is not possible to reduce LDL-C in adults with hypercholesterolemia, including HeFH.
IMPORTANT SAFETY INFORMATION
NEXLIZET and NEXLETOL are contraindicated in patients with a prior hypersensitivity to bempedoic acid or ezetimibe or any of the excipients. Serious hypersensitivity reactions including anaphylaxis, angioedema, rash, and urticaria have been reported.Hyperuricemia: Bempedoic acid, a component of NEXLIZET and NEXLETOL, may increase blood uric acid levels, which may lead to gout. Hyperuricemia may occur early in treatment and persist throughout treatment, returning to baseline following discontinuation of treatment. Assess uric acid levels periodically as clinically indicated. Monitor for signs and symptoms of hyperuricemia, and initiate treatment with urate-lowering drugs as appropriate.
Tendon Rupture: Bempedoic acid, a component of NEXLIZET and NEXLETOL, is associated with an increased risk of tendon rupture or injury. Tendon rupture may occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders. Discontinue NEXLIZET or NEXLETOL at the first sign of tendon rupture. Consider alternative therapy in patients who have a history of tendon disorders or tendon rupture.
The most common adverse reactions in the primary hyperlipidemia trials of bempedoic acid, a component of NEXLIZET and NEXLETOL, in ≥2% of patients and greater than placebo were upper respiratory tract infection, muscle spasms, hyperuricemia, back pain, abdominal pain or discomfort, bronchitis, pain in extremity, anemia, and elevated liver enzymes.
Adverse reactions reported in ≥2% of patients treated with ezetimibe (a component of NEXLIZET) and at an incidence greater than placebo in clinical trials were upper respiratory tract infection, diarrhea, arthralgia, sinusitis, pain in extremity, fatigue, and influenza.
In the primary hyperlipidemia trials of NEXLIZET, the most commonly reported adverse reactions (incidence ≥3% and greater than placebo) observed with NEXLIZET, but not observed in clinical trials of bempedoic acid or ezetimibe, were urinary tract infection, nasopharyngitis, and constipation.
The most common adverse reactions in the cardiovascular outcomes trial for bempedoic acid, a component of NEXLIZET and NEXLETOL, at an incidence of ≥2% and 0.5% greater than placebo were hyperuricemia, renal impairment, anemia, elevated liver enzymes, muscle spasms, gout, and cholelithiasis.
Discontinue NEXLIZET or NEXLETOL when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus. Because of the potential for serious adverse reactions in a breast-fed infant, breastfeeding is not recommended during treatment with NEXLIZET or NEXLETOL.
Report pregnancies to Esperion Therapeutics, Inc. Adverse Event reporting line at 1-833-377-7633.
Please see full Prescribing Information for NEXLIZET and NEXLETOL.
About Esperion Therapeutics
Esperion Therapeutics, Inc. is a commercial-stage biopharmaceutical company dedicated to developing and delivering innovative cardiometabolic and rare/orphan disease therapies. The Company leverages deep domain expertise in ACLY biology to develop and commercialize transformative medicines for patients worldwide. Esperion currently markets two oral, once-daily, non-statin therapies for patients struggling to maintain their low-density lipoprotein cholesterol (LDL-C) levels and are at risk of cardiovascular disease.With a broad U.S. commercial infrastructure and global approvals across more than 40 countries, Esperion is well positioned to serve as a partner-of-choice for global innovators seeking U.S. market access through acquisition, in-license, co-promotion and revenue share opportunities. In tandem, the Company is advancing its leadership in ACLY biology to build a diversified pipeline of novel product candidates, including treatments for Primary Sclerosing Cholangitis and renal diseases. For more information, visit esperion.com and follow Esperion on LinkedIn and X.
Forward-Looking Statements
This press release contains forward-looking statements that are made pursuant to the safe harbor provisions of the federal securities laws, including statements regarding marketing strategy and commercialization and business development plans, current and planned operational expenses, expected profitability, future operations, commercial products, clinical development, including the timing, designs and plans for the CLEAR Outcomes study and its results, plans for potential future product candidates, financial condition and outlook, including expected cash runway and profitability, and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “suggest,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions. Any express or implied statements contained in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Forward-looking statements involve risks and uncertainties that could cause Esperion’s actual results to differ significantly from those projected, including, without limitation, the net sales, profitability, and growth of Esperion’s commercial products, clinical activities and results, supply chain, commercial development and launch plans, business development, the outcomes and anticipated benefits of legal proceedings and settlements, and the risks detailed in Esperion’s filings with the Securities and Exchange Commission. Any forward-looking statements contained in this press release speak only as of the date hereof, and Esperion disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this press release, other than to the extent required by law.Esperion Contact Information:
Investors:
Alina Venezia
investorrelations@esperion.com
(734) 887-3903Media:
Tiffany Aldrich
corporateteam@esperion.com
(616) 443-8438Continue Reading

Samsung and Netflix Offer Exclusive “Stranger Things” Theme for Galaxy – Samsung Mobile Press
As the door closes on “Stranger Things,” Samsung Electronics and Netflix are giving…
Continue Reading

Teva Pharmaceutical Industries Ltd. – Teva to Present at the 44th Annual J.P. Morgan Healthcare Conference: Pivot to Growth Strategy Delivering Growth and Transforming through Innovation
Teva to Present at the 44th Annual J.P. Morgan Healthcare Conference: Pivot to Growth Strategy Delivering Growth and Transforming through Innovation
Richard Francis, Teva’s President and CEO, will present at the 44th Annual J.P. Morgan Healthcare Conference on Tuesday, January 13, 2026, at 8:15 A.M. Pacific Time (11:15 A.M. Eastern Time)
TEL AVIV, Israel, Jan. 11, 2026 (GLOBE NEWSWIRE) — Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) will present its ongoing transformation and expected growth trajectory through 2030 at the 44th Annual J.P. Morgan Healthcare Conference. Teva’s President and Chief Executive Officer, Richard Francis, will meet with investors and present the Company’s milestones achieved in 2025, transformative initiatives, and forward-looking outlook for 2026 and beyond.
Presentation Highlights:
- Pivot to Growth Strategy Progress: Teva is accelerating its Pivot to Growth strategy, focusing on transforming into a leading innovative biopharmaceutical company through late-stage innovative pipeline, fueled by its world-class generics business.
- Innovation at the Heart of Teva’s Transformation: Teva’s key innovative brands – AUSTEDO®, AJOVY® and UZEDY® – are already driving its growth and reshaping Teva’s financial outlook. Teva’s clinical pipeline assets – olanzapine LAI, DARI (ICS/SABA), duvakitug (anti-TL1A), emrusolmin, and anti-IL-15 – are expected to drive Teva’s long-term growth trajectory and further Teva’s transformation.
- 2025 Performance: Teva to provide its expected 2025 financial performance.
- 2026 and Beyond: In addition, Teva to provide forward-looking outlook for 2026 and beyond, underlining disciplined capital allocation and a commitment to securing an investment-grade credit rating.
Expected 2025 Performance
$ billions, except EPS or as noted
2025 Outlook
Expected 2025 performance relative to Outlook (excluding duvakitug milestones)
Additional contribution from expected duvakitug milestones
Revenues*
$16.8 – $17.0
Lower point of the range
$500M
Operating Margin
~26.2% – 27.1%
Mid to high point of the range
~80%-85%
Adjusted EBITDA
$4.8 – $5.0
Midpoint of the range
~$400M-$430M
Tax Rate
15%-18%
Lower point of the range
Diluted EPS ($)
2.55 – 2.65
Higher point of the range
Free Cash Flow**
$1.6 – $1.9
Higher point of the range
~$500M
Net leverage
~2.5x – 2.9x
Midpoint of the range
~2.5x
Path to achieving 2027 targets and additional 2030 targets$billionsor as noted
2026
2027
2030
Revenues*
Flat to slightly down vs. 2025
Low-single digit growth
Mid-single digit CAGR
Operating Profit
Growing vs. 2025
30%
>30%
Adjusted EBITDA
Growing vs. 2026
Growing
Free Cash Flow**
Growing vs. 2025
>$2.7
>$3.5
Net Leverage
~2.0-2.2x
<2x
<2x
Cumulative Transformation Programs Savings
~$450M-500M
~$700M
Note: 2026 commentary compared to 2025 results excluding duvakitug milestones, except for net debt leverage calculation.
* Revenues presented on a GAAP basis; all other metrics presented on a non-GAAP basis.
** Free Cash Flow includes cash flow generated from operating activities net of capital expenditures and deferred purchase price cash component collected for securitized trade receivables.To access a live webcast of the presentation, visit Teva’s Investor Relations website at: https://ir.tevapharm.com/Events-and-Presentations.
An archived version of the webcast will be available within 24 hours after the end of the live discussion and will be accessible for up to 30 days.
About Teva
Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) is transforming into a leading innovative biopharmaceutical company, enabled by a world-class generics business. For over 120 years, Teva’s commitment to better health has never wavered. From innovating in the fields of neuroscience and immunology to providing complex generic medicines, biosimilars and pharmacy brands worldwide, Teva is dedicated to addressing patients’ needs, now and in the future. At Teva, We Are All In For Better Health. To learn more about how, visit www.tevapharm.com.Non-GAAP Financial Measures
This press release includes certain non-GAAP financial measures as defined by SEC rules. Management believes that such non-GAAP financial measures provide useful information to investors to facilitate their understanding of our business because the non-GAAP financial measures are used by Teva’s management and board of directors, in conjunction with other performance metrics, to evaluate the operational performance of the company, to compare against the company’s work plans and budgets, and ultimately to evaluate the performance of management; the company’s annual budgets are prepared on a non-GAAP basis; and senior management’s annual compensation is derived, in part, using these non-GAAP measures. Investors should consider the non-GAAP financial measures in addition to, and not as replacements for, or superior to, measures of financial performance prepared in accordance with GAAP. In the case of the non-GAAP financial measures disclosed in this press release, we are not providing comparable forward looking guidance for GAAP financial measures or a quantitative reconciliation of forward-looking non-GAAP financial measures to the most directly comparable GAAP measure because we are unable to predict with reasonable certainty the ultimate outcome of certain significant items including, but not limited to, the amortization of purchased intangible assets, legal settlements and loss contingencies, impairment of long-lived assets and goodwill impairment, without unreasonable effort. These items are uncertain, depend on various factors, and could be material to our results computed in accordance with GAAP.Teva Cautionary Note Regarding Forward Looking Statements
This Press Release and the presentation at the conference may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding our financial guidance, which are based on management’s current beliefs and expectations and are subject to substantial risks and uncertainties, both known and unknown, that could cause our results, performance or achievements to differ significantly from that expressed or implied by such forward-looking statements. These forward-looking statements include statements concerning our plans, strategies, objectives, future performance and financial and operating targets, and any other information that is not historical information. You can identify these forward-looking statements by the use of words such as “should,” “expect,” “anticipate,” “estimate,” “target,” “may,” “project,” “guidance,” “intend,” “plan,” “believe,” “outlook” and other words and terms of similar meaning and expression in connection with any discussion of future operating or financial performance. Important factors that could cause or contribute to such differences include risks relating to: our ability to successfully compete in the marketplace, including: that we are substantially dependent on our generic products; our ability to develop and commercialize additional pharmaceutical products; competition for our innovative medicines; our ability to achieve expected results from investments in our product pipeline; our ability to successfully execute on our Pivot to Growth strategy, including to expand our innovative and biosimilar medicines pipeline and profitably commercialize the innovative medicines and biosimilar portfolio, whether organically or through business development, to sustain and focus our portfolio of generic medicines, and to execute on our organizational transformation and to achieve expected cost savings; and the effectiveness of our patents and other measures to protect our intellectual property rights; our significant indebtedness, which may limit our ability to incur additional indebtedness, engage in additional transactions or make new investments; our business and operations in general; compliance, regulatory and litigation matters; other financial and economic risks; and other factors discussed in this document, in our Quarterly Report on Form 10-Q for the third quarter of 2025 and in our Annual Report on Form 10-K for the year ended December 31, 2024, including in the sections captioned “Risk Factors” and “Forward-looking Statements.” Forward-looking statements speak only as of the date on which they are made, and we assume no obligation to update or revise any forward-looking statements or other information contained herein, whether as a result of new information, future events or otherwise. You are cautioned not to put undue reliance on these forward-looking statements.
Continue Reading

Samsung Launches New Tool To Help Consumers Find Their Ideal Washing Machine – Samsung Global Newsroom
Samsung Electronics today announced the launch of “Help Me Choose,” a digital tool designed to guide consumers in selecting the ideal laundry solution based on their individual needs and lifestyle preferences.
With just a few clicks, the tool evaluates suitability across product types including washers, dryers, washer & dryer sets and all-in-one combos, empowering users to make smarter decisions based on real-time product recommendations.
“Today’s consumers have a wide array of laundry needs and preferences, and that means there is no one-size-fits-all solution,” said Sang Jik Lee, Executive Vice President and Head of Sales & Marketing Team for Digital Appliances (DA) Business at Samsung Electronics. “By simplifying the decision-making process and offering a more intuitive product discovery journey, the ‘Help Me Choose’ tool makes it easy to find the best fit.”
Designed for a Range of Modern Lifestyles
Samsung has continually expanded its washing machine portfolio to meet the needs of diverse markets and lifestyles — from compact models for people living alone to high-capacity appliances for larger households.
When consumers shopping online, however, the wide range of available models can sometimes make the selection process overwhelming. Reflecting the growing trend of online shopping for home appliances and the expanding variety of consumer lifestyles worldwide, the new tool guides users through a series of lifestyle-based questions, such as how big their household is, how often they do laundry in a week, how much space is available and what values, like energy or time saving, are most important to them. Based on these responses, users can receive tailored recommendations from Samsung’s lineup of washing machines available in their region.
For example, if someone lives alone, owns a pet and values space efficiency, the tool can recommend the Bespoke AI Laundry Combo. This is because it provides an efficient laundry experience by completing both washing and drying in a single unit, avoiding the need for two separate machines, while also offering the Pet Care and AI Wash & Dry1 cycles.
Availability
Help Me Choose will roll out on Samsung.com in select global markets starting in January, with broader availability to follow.
Samsung also plans to expand the experience to additional product categories starting in the first half of 2026, helping consumers make confident, informed purchase decisions more conveniently.
Continue Reading

Doha Was a Deal, Not a Model
The suggestion of Zalmay Khalilzad to make Pakistan pursue an agreement of the Doha format with Kabul is pegged on a retrocedent vision of the region. It assumes that Pakistan still needs a tremendous bargain to prove its goodwill, and it…
Continue Reading

Samsung and Netflix Offer Exclusive ‘Stranger Things’ Theme for Galaxy – Samsung Global Newsroom
As the door closes on “Stranger Things,” Samsung Electronics and Netflix are giving fans a chance to celebrate the final season of the iconic show that has shaped pop culture for nearly a decade. Starting Jan. 12, Samsung…
Continue Reading
Ohio State 89-76 Maryland (Jan 11, 2026) Game Recap – ESPN
- Ohio State 89-76 Maryland (Jan 11, 2026) Game Recap ESPN
- Jaloni Cambridge leads No. 19 Buckeyes past No. 8 Terps The Baltimore Banner
- No. 8 Terps Fall 89-76 In Big Ten Bout With No. 19 Buckeyes University of Maryland Athletics
- The Ohio State and…
Continue Reading
Ohio State 89-76 Maryland (Jan 11, 2026) Game Recap – ESPN
- Ohio State 89-76 Maryland (Jan 11, 2026) Game Recap ESPN
- Ohio St Maryland Basketball couriernews.com
- Ohio State vs. Maryland prediction: Why Maryland is favored to win women’s college basketball matchup [1/11/2026] dimers.com
- No. 19 Ohio St. 89,…
Continue Reading