- Avian flu (bird flu): near Penicuik, Scottish Borders (AIV 2026/04) The Scottish Government
- Hong Kong suspends importation of chicken and eggs from Perth due to bird flu STV News
- Poultry farm chickens to be culled over bird flu in Newark BBC
- Bird…
Author: admin
-

Avian flu (bird flu): near Penicuik, Scottish Borders (AIV 2026/04) – The Scottish Government
-
Wang Yi Holds a Phone Conversation with Foreign Minister of Somalia Abdisalam Dhaay_Ministry of Foreign Affairs of the People’s Republic of China
On January 11, 2026 local time, during his visit to Africa, Member of the Political Bureau of the CPC Central Committee and Foreign Minister Wang Yi held a phone conversation with Foreign Minister of Somalia Abdisalam Dhaay.
Wang Yi asked…
Continue Reading
-

My top 5 phones of 2025 – George
So, this is going to be a weirdly motivated list, but hear me out. Finding 5 phones to really like in a year – in 2025 and in our line of work – is no trivial task. Take Ro, for example – he only made it to 4, as did Sagar, and Ivan has 3…
Continue Reading
-

McDavid extends point streak to 18 for Oilers, ‘very motivated to be the best player’
Consistency has been the key to McDavid’s success this season, as he’s been held off the score sheet only six times. The last time he did not have a point was on Dec. 2, a 1-0 loss to the Minnesota Wild.
He has 78 points (30 goals, 48…
Continue Reading
-

Donald Trump won’t take Greenland by force, Lord Mandelson says
 Getty Images
Getty ImagesDenmark has warned any military intervention on Greenland would destroy the Nato alliance (file photo) US President Donald Trump would not “land on Greenland and take it by force”, Lord Mandelson has said.
The former UK ambassador to…
Continue Reading
-
New Hampshire vaccine laws unchanged despite CDC cuts
New Hampshire’s long-established requirements on vaccines needed to attend public school have not been altered despite recent cutbacks in federal guidelines announced by Robert Kennedy Jr., but several proposed bills in Concord…
Continue Reading
-

Google’s AI Inbox could be a glimpse of Gmail’s future
This week, Google announced a new AI Inbox view for Gmail that replaces the traditional list of emails with an AI-generated list of to-dos and topics to track based on what’s in your inbox. It’s not widely available yet, but I have access,…
Continue Reading
-
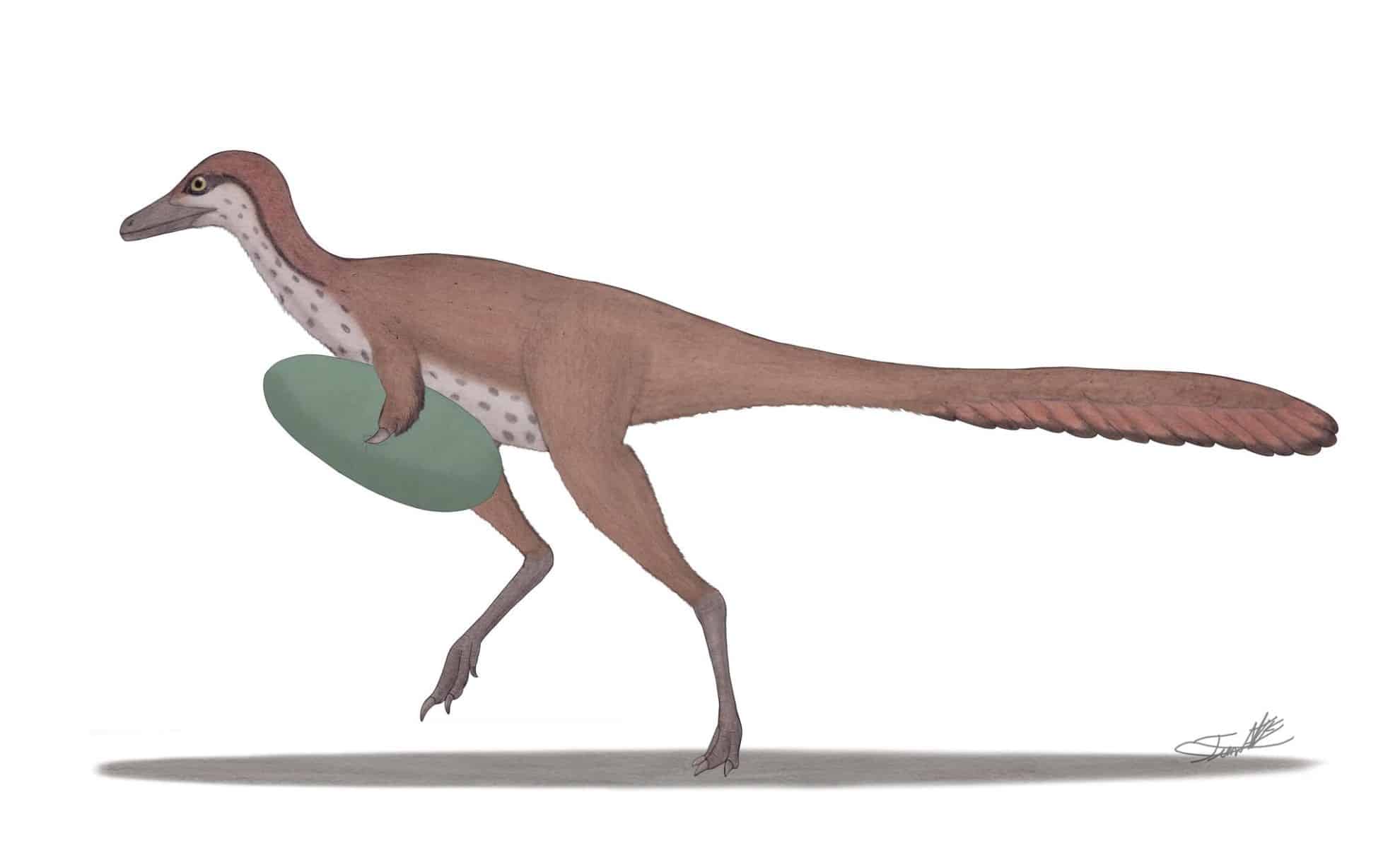
This Tiny Dinosaur Had a Weaponized Hand Built to Steal Eggs
A small dinosaur with a giant claw and a bizarre hand structure is changing how scientists think about prehistoric predators. The newly described Manipulonyx reshetovi, found in the Gobi Desert, likely used its specialized forelimb to grip and…
Continue Reading
-

Lincolnshire food waste collections to be rolled out in county
Residents will also be provided with an initial roll of caddy liners to help them get started, the council said.
Waste such as plate scrapings, peelings, tea bags, coffee grounds and other items can be placed in the liner, tied up, and transferred to the outdoor caddy for the weekly collection.
Collected food waste will then be sent to a specialist anaerobic digestion facility, where it will be converted into renewable energy and nutrient-rich fertiliser, helping to support local farming and power homes and businesses.
The council’s cabinet member for environment and waste, Councillor Rhys Baker, said: “Many residents are already enthusiastic recyclers and I am sure they will embrace this new system to see where it can take us.
“This is a simple change with a powerful impact. Together, we can reduce waste, generate clean energy, and make Lincolnshire greener for generations to come.”
East Lindsey, Boston and South Holland are expected to roll out their own food waste collection service in the autumn.
Continue Reading
-
Trump Firm Sharpens Saudi Property Focus as Prince Opens Market – Bloomberg.com
- Trump Firm Sharpens Saudi Property Focus as Prince Opens Market Bloomberg.com
- Trump Organization deepens Gulf push with $10bn in Saudi projects Financial Times
- Dar Global and Trump Organisation launch luxury project in Riyadh’s Diriyah MSN
- Dar Global and Trump Organization launch $10 billion Saudi developments By Reuters Investing.com
Continue Reading