Abilene, Texas – The Centenary men’s basketball team defeated the McMurry University War Hawks by a score of 94-89 in a Southern Collegiate Athletic Conference contest on Friday evening inside Kimbrell Arena.
The…
Abilene, Texas – The Centenary men’s basketball team defeated the McMurry University War Hawks by a score of 94-89 in a Southern Collegiate Athletic Conference contest on Friday evening inside Kimbrell Arena.
The…
On 4 December 2025, the Pan American Health Organization/World Health Organization (PAHO/WHO) issued an alert regarding the possibility of early or more intense activity of respiratory viruses during the 2025-26 season, as compared to previous…
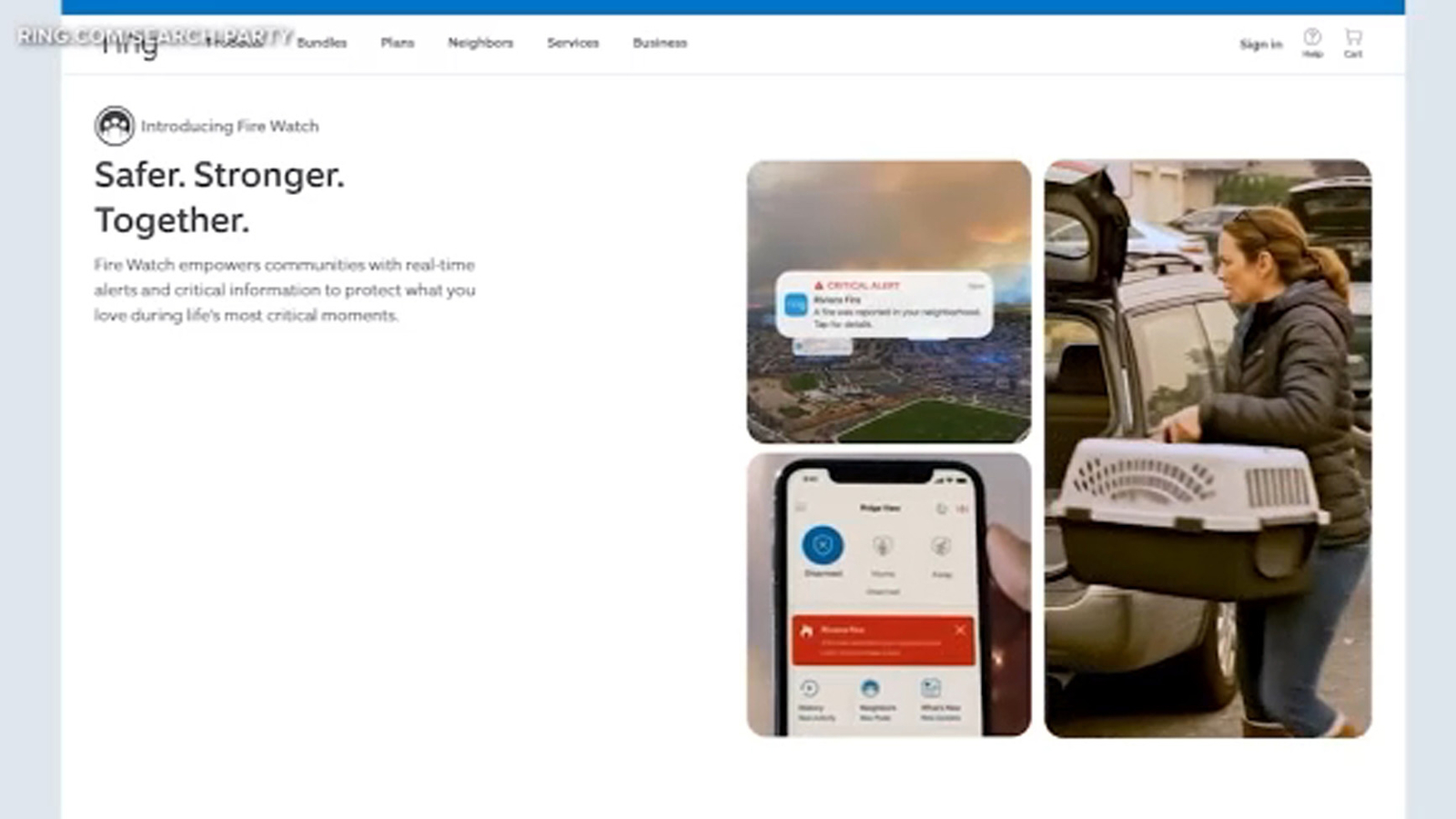
LOS ANGELES (KABC) — Much of the Pacific Palisades neighborhood where Ring founder Jamie Siminoff once lived – including the garage where he invented the Ring video doorbell – was destroyed in the devastating wildfire.
Siminoff’s own home was…


EVANSTON, Ill. — The Maryland wrestling team (4–2, 0–1 B1G) fell to Northwestern (3–1, 1–0 B1G),…

A man and woman who were shot by an immigration agent in Portland, Oregon, on Thursday had ties to the Venezuelan gang Tren de Aragua, officials said.
In a news conference on Friday, Portland Police Chief Bob Day confirmed that both “do have some…

» Michigan dominated in the faceoff dot, resulting in a 39-19 advantage.
» Upperclassmen Jayden Perron and T.J. Hughes each contributed a pair of assists.
» Nick Moldenhauer finished with two points after burying the empty-net marker.
ANN…