Opticians are starting a challenge to run, walk and cycle 6,214 miles (10,000km) in 2026 – one kilometre for each person estimated to be living with sight loss across Devon and Cornwall.
Optician Tom Mannering said: “This number has real meaning…

Opticians are starting a challenge to run, walk and cycle 6,214 miles (10,000km) in 2026 – one kilometre for each person estimated to be living with sight loss across Devon and Cornwall.
Optician Tom Mannering said: “This number has real meaning…

#7/6 Maryland (14-1, 2-1) vs. Indiana (11-4, 0-3)
January 4, 2026
6 PM ET
XFINITY…

With the busy holiday season officially behind us, a lot of people are settling back into their regular routines — which, ideally, includes carving out some time to relax with a good book. If you read on a Kindle or use the Kindle app,…

 Carolyn Harris
Carolyn HarrisAn MP who lost nearly 10 stone on Mounjaro has said people who take unregulated weight-loss jabs are “playing Russian roulette…
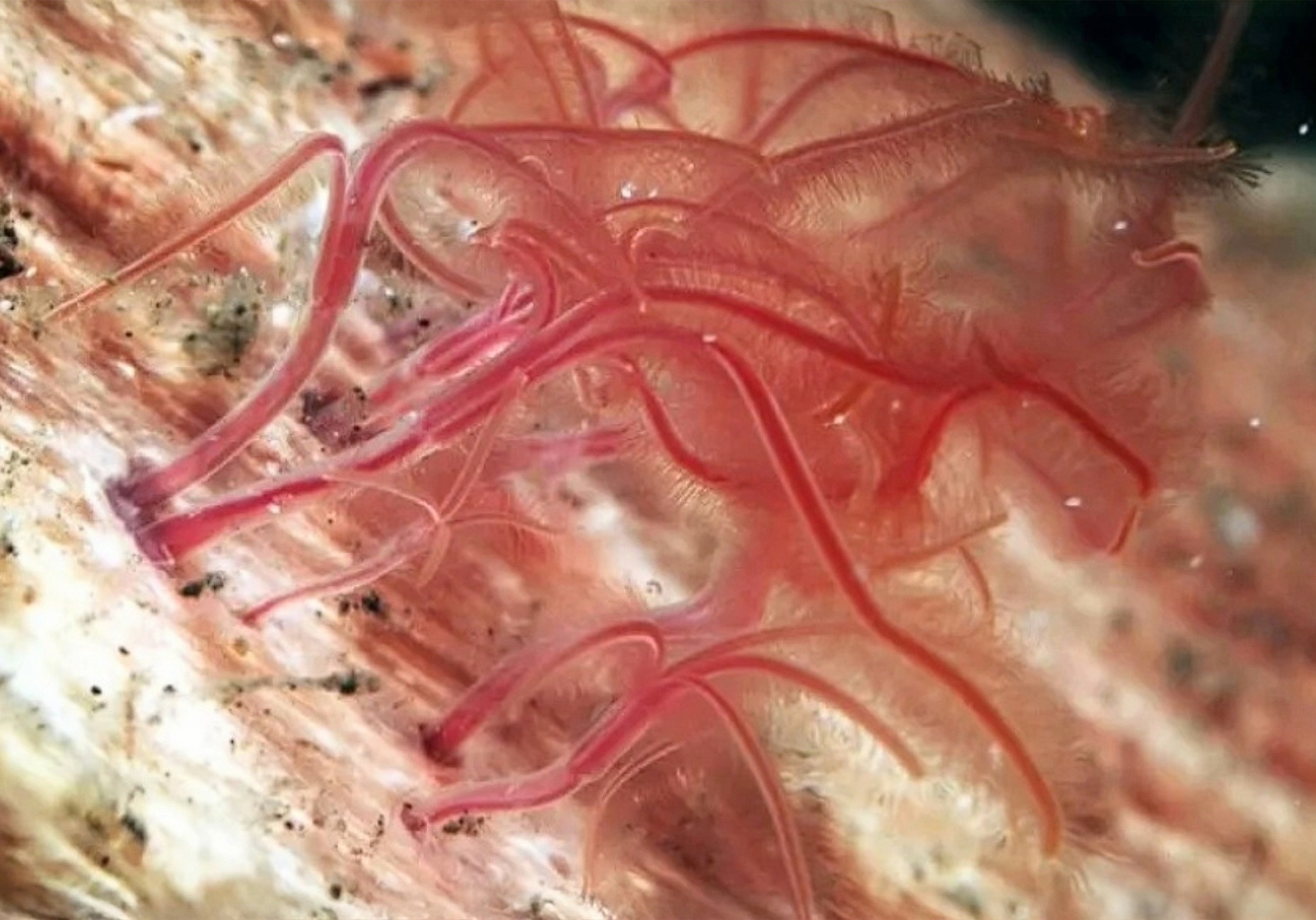
Horror movies are full of monsters you never quite see. Lately, the deep Pacific off British Columbia has its own version of that: a crucial worm that simply isn’t showing up.
The no-show is Osedax – nicknamed the “zombie worm” – a tiny,…


Quantum Biopharma has completed dosing in two toxicology studies requested by the U.S. Food and Drug Administration (FDA) that aim to support the launch of clinical studies of Lucid-MS, an experimental treatment for multiple sclerosis (MS)…
A decade after Saudi Arabia and the United Arab Emirates led a joint military campaign to curb Iran’s influence in Yemen, the two allies now find themselves pitted against each other there.
…
By Matthew Stenger
Posted: 1/2/2026 10:04:00 AM
Last Updated:
In a phase III trial (inMIND) reported in The Lancet, Sehn et al found that the addition of the CD19-targeted Fc-enhanced monoclonal antibody tafasitamab to lenalidomide plus rituximab improved progression-free survival in patients with relapsed or refractory follicular lymphoma.
Study Details
In the global double-blind trial, 548 patients from sites in North America, Europe, and the Asia-Pacific region were randomly assigned between April 2021 and August 2023 to receive up to twelve 28-day cycles of tafasitamab at 12 mg/kg on days 1, 8, 15, and 22 of cycles 1 to 3 and days 1 and 15 of cycles 4 to 12 (n = 273) or placebo (n = 275), with both groups receiving lenalidomide at 20 mg/day on days 1 to 21 of cycles 1 to 12 and rituximab at 375 mg/m² on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 2 to 5. In total, 80% of patients were White. The primary endpoint was investigator-assessed progression-free survival in the intention-to-treat population.
Key Findings
Median follow-up was 14.3 months (95% confidence interval [CI] = 11.8–15.0 months) in the tafasitamab group and 14.1 months (95% CI = 11.5–15.0 months) in the control group. Median progression-free survival on investigator assessment was 22.4 months (95% CI = 19.2 months to not evaluable) in the tafasitamab group vs 13.9 months (95% CI = 11.5–16.4 months) in the control group (hazard ratio [HR] = 0.43, 95% CI = 0.32–0.58, P < .0001).
On independent review committee assessment, median progression-free survival was not reached (95% CI = 19.3 months to not evaluable) in the tafasitamab group vs 16.0 months (95% CI = 13.9–21.1 months) in the control group (HR = 0.41, 95% CI = 0.29–0.56, P < .0001).
Death occurred in 15 patients in the tafasitamab group and 23 patients in the control group. Full analysis of overall survival is planned at 5 years of follow-up.
Grade 3 to 4 adverse events occurred in 71% of the tafasitamab group vs 69% of the control group. The most common were neutropenia (40%), pneumonia (8%), COVID-19 (6%), and thrombocytopenia (6%) in the tafasitamab group, and neutropenia (38%), thrombocytopenia (7%), anemia (6%), and pneumonia (5%) in the control group. Serious adverse events occurred in 36% vs 32% of patients, most commonly pneumonia in both groups (8% vs 5%). Adverse events led to the discontinuation of treatment in 11% vs 7% of patients.
The investigators concluded: “The addition of tafasitamab to lenalidomide and rituximab resulted in a statistically significant and clinically meaningful improvement in progression-free survival, with an acceptable safety profile in patients with relapsed or refractory follicular lymphoma. This combination represents a potential new standard-of-care treatment.”
Laurie H. Sehn, MD, of BC Cancer Centre for Lymphoid Cancer and The University of British Columbia, Vancouver, Canada, is the corresponding author for The Lancet article.
Disclosure: The study was funded by Incyte. For full disclosures of the study authors, visit thelancet.com.

See what the new year has in store for space science and exploration. The Planetary Society’s calendar of space events for 2026 outlines all the most spectacular launches, space mission milestones, and celestial events to watch out for over…