T-R FILE PHOTO
Scout prepares to get on the water upon her November arrival at Riverside Cemetery. Serving as a new companion for fellow goose Frankie, Scout was found dead last Sunday and has…
Author: admin
-

Riverside Cemetery goose Scout passes unexpectedly | News, Sports, Jobs
-
PSX surges past 178,000 mark as KSE-100 Index gains over 2,300 points
The Pakistan Stock Exchange (PSX) reached new heights on Friday, with the benchmark KSE-100 Index crossing the 178,000 level for the first time in its history.
At 11:10 am, the KSE-100 Index was trading at 178,715.38, up by 2359.89 points, or 1.34%, reflecting broad-based buying interest in key sectors, including automobile assemblers, cement, commercial banks, fertilizer, oil and gas exploration, OMCs, power generation, and refineries. Index-heavy stocks such as HUBCO, ARL, MARI, OGDC, PPL, POL, PSO, HBL, NBP, and UBL all saw gains.
Pakistan’s headline inflation rate for December 2025 stood at 5.6% on a year-on-year basis, in line with the Ministry of Finance’s estimated range of 5.5-6.5%.
The country also saw a reversal in foreign investment trends in December, with net inflows of $20 million in short-term local government bonds, up from $42.2 million in outflows the previous month. Foreign investors invested $77.29 million into treasury bills, although $57.27 million was divested during the same period.
Meanwhile, Pakistan’s total liquid foreign exchange reserves stood at $21.012 billion as of December 26, 2025, showing a slight decline from $21.023 billion recorded a week earlier.
The PSX had a strong start to the year, with a broad rally that lifted all major indices. On Thursday, the KSE-100 Index posted a sharp gain of 2,301.17 points, or 1.32%, closing at 176,355.49 points, signaling strong market optimism for 2026.
International markets also began 2026 on a positive note, though trading was thin due to holiday breaks. In Asia, MSCI’s broad index of shares outside Japan rose 0.66%, while Hong Kong’s Hang Seng Index gained 1.24%. U.S. futures, including S&P 500 and Nasdaq, also saw gains, while European futures showed mixed results.
Continue Reading
-

Burgundy bins in North Lincolnshire to be recycled and replaced
Existing household burgundy bins will be collected and recycled as part of North Lincolnshire Council’s “simpler” recycling system.
New bins will be sent out later this month which residents can use for dry recycling, including plastic, metal, glass, card and paper.
The authority said the new kerbside collection system will make it easier for residents to “recycle more and waste less”.
Council leader Rob Waltham said the old containers would be “recycled responsibly”.
Under the new system, 70,000 larger recycling bins will be distributed across North Lincolnshire.
A garden and food waste bin will be collected weekly with a general waste bin collected fortnightly, the authority said.
Deputy council leader Neil Poole said: “We’re upgrading the system and making it easier for residents.
“Every old bin collected is turned back into useful raw material, helping manufacturers around the country and cutting waste at the same time.”
The council said the existing bins would be turned into pellets which can be used by manufacturers to create new products.
Deliveries of the new bins and collection of the existing ones will begin shortly after the council receives its first shipment in January.
Residents who would prefer to keep their current recycling bin can opt out of the scheme through an online form.
Continue Reading
-

Burgundy bins in North Lincolnshire to be recycled and replaced
Under the new system, 70,000 larger recycling bins will be distributed across North Lincolnshire.
A garden and food waste bin will be collected weekly with a general waste bin collected fortnightly, the authority said.
Deputy council leader Neil Poole said: “We’re upgrading the system and making it easier for residents.
“Every old bin collected is turned back into useful raw material, helping manufacturers around the country and cutting waste at the same time.”
The council said the existing bins would be turned into pellets which can be used by manufacturers to create new products.
Deliveries of the new bins and collection of the existing ones will begin shortly after the council receives its first shipment in January.
Residents who would prefer to keep their current recycling bin can opt out of the scheme through an online form.
Continue Reading
-

Plans for car themed play area on old Daimler factory site
Play equipment based on a motoring theme could be built on the site of the former Daimler car factory if plans are approved.
The play area would be part of a public plaza in Sandy Lane, Coventry, which would provide spaces for public gatherings and meetings.
Proposals will accompany the development of up to 250 homes that will be built on the site after plans for the homes were approved in June.
Developers Dandara Central said the plaza aimed to be multi-functional, with trees and planting beds to improve the frontage of the Daimler Powerhouse building.
The features of the play area would “encourage play and movement” while linking back to the site’s heritage, the developers added.
Links between the site and the Coventry Canal will be created under the plan with a direct connection for pedestrians and cyclists between both the plaza and nearby properties.
“These connections will benefit existing residents in the local area by re-opening a crucial north-south walking and cycling connection north of the city centre,” developers said.
The Daimler building was one of the first car plants in the country and much of the site was destroyed in the Blitz.
The firm was taken over by Coventry Climax to test out forklift trucks and it designed the UK’s first forklift truck in 1946.
Continue Reading
-

Otterbourne pantomime’s ‘relaxed’ performance ‘accessible to all’
Tom Horscroft, an audience member at one of the dress rehearsals at the 240-person capacity venue, said the show was “perhaps not quite so scary in certain parts”.
“But it really gets the kids involved and the audience are really on board, with…
Continue Reading
-
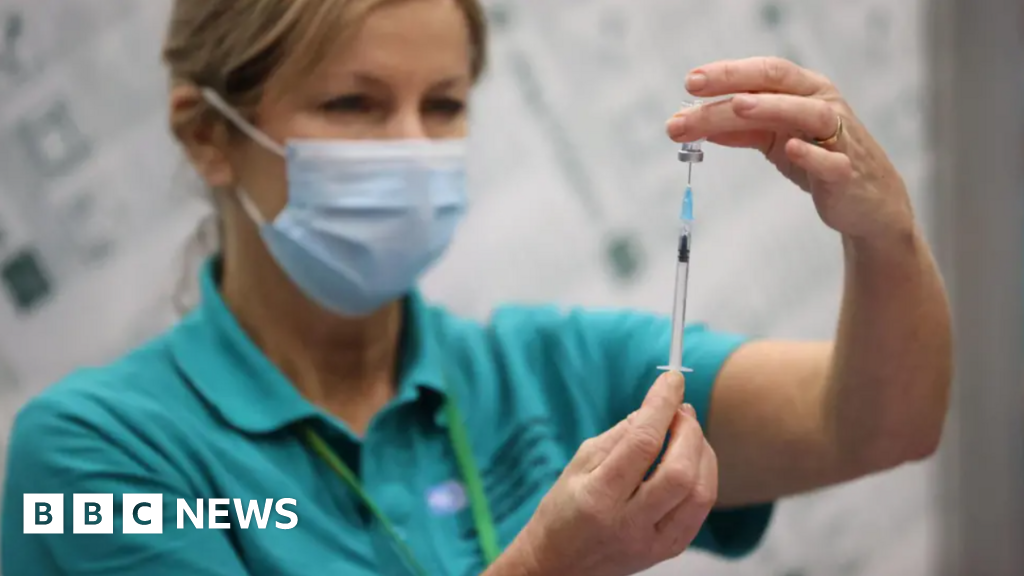
South West children offered free NHS chickenpox vaccine
Children in the South West can now get what has been described as a “historic” free vaccination against chickenpox from the NHS, health bosses say.
The Joint Committee on Vaccination and Immunisation, which advises UK health departments,…
Continue Reading
-

Drones could save vaccine waste in Rwanda, Birrmingham University researchers say
The problem with vaccines is they need to be refrigerated. In some of the more remote clinics, without easy road access or reliable power supplies, they are vulnerable, especially if there are issues with the on site fridges.
If the power fails…
Continue Reading
-

Drones could save vaccine waste in Rwanda, Birrmingham University researchers say
In parts of Africa almost a quarter of vaccine doses are thrown away because they have not been stored at the right temperature.
Research from the University of Birmingham suggests delivery by drone could stop this.
In Rwanda, commercial drone…
Continue Reading
-
The Sky This Week from December 2 to 9: The Quadrantid meteor shower peaks – Astronomy Magazine
- The Sky This Week from December 2 to 9: The Quadrantid meteor shower peaks Astronomy Magazine
- January’s full wolf supermoon and the Quadrantid meteor shower will start off the new year CNN
- The Best Meteor Showers in 2026 Sky & Telescope
- The…
Continue Reading