This was a year that tested the resilience of the Huntington’s disease (HD) community, with 2025 being remembered for landmark highs followed by disappointing lows shadowed in confusion. Data from clinical trials produced the strongest…
Author: admin
-

Lchashen wagon: A 3,500-year-old covered wagon that transported a deceased chief to the next world
QUICK FACTS
Name: Lchashen wagon
What it is: An oak wagon
Where it is from: Lchashen village, Armenia
When it was made: Circa 1500 B.C.
Covered wagons are often associated with the Old West. But the best-preserved example of an ancient covered wagon…
Continue Reading
-

13 must-see moon events in 2026 — Eclipses, supermoons, conjunctions and more
A bevy of supermoons, a dramatic total solar eclipse and a “blood moon” total lunar eclipse are some of the highlights of the coming lunar year, which will also include plenty of beautiful close conjunctions of the crescent moon and…
Continue Reading
-
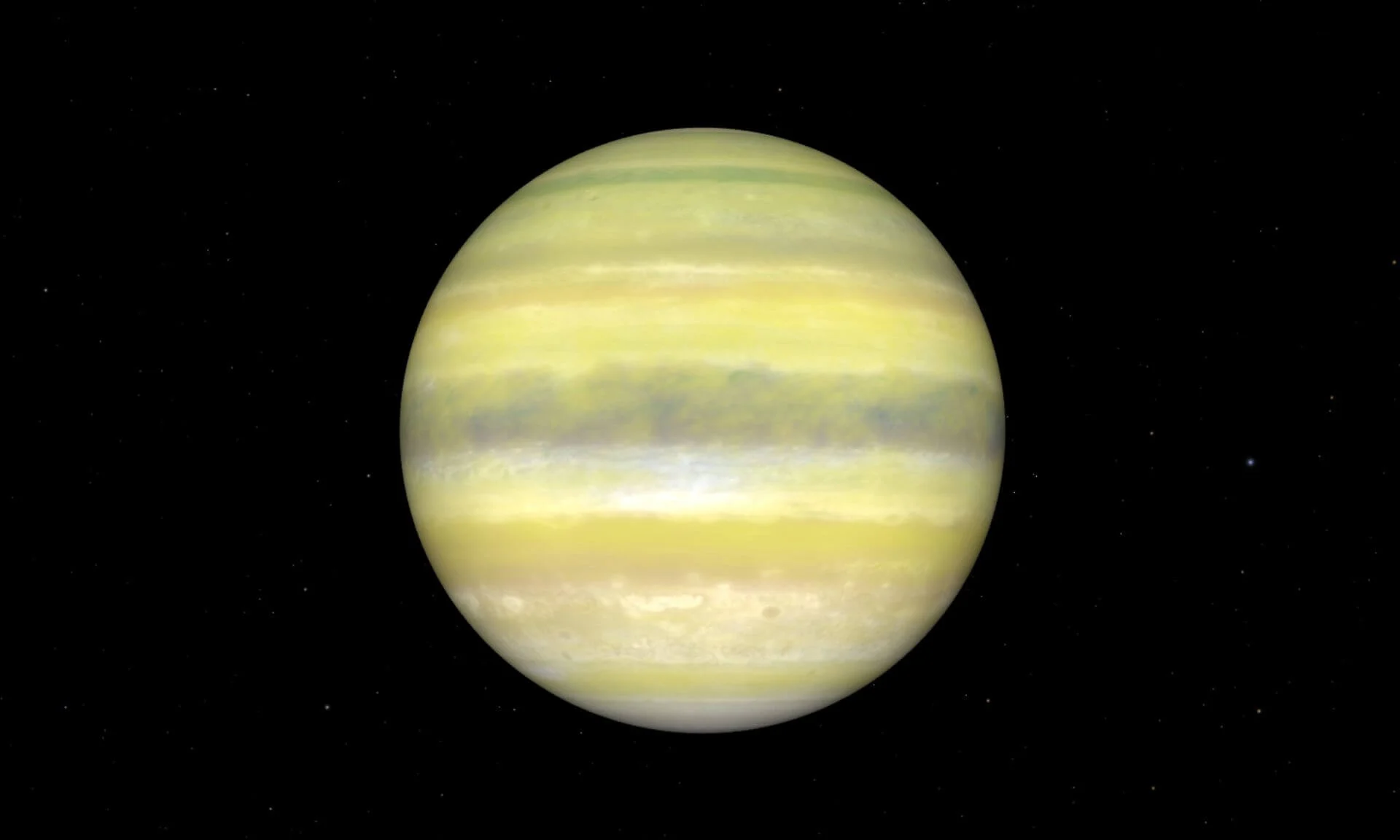
Webb reveals a hazy world that shows not all planet twins are identical
Astronomers from the Trottier Institute for Research on Exoplanets (IREx) at the Université de Montréal have used the James Webb Space Telescope (JWST) to uncover the surprising nature of a world that looked, at…
Continue Reading
-

New Year’s Eve TV guide: Including the Jonas Brothers and Huntr/x
A Jonas family New Year’s Eve in Wyckoff, N.J., was always boisterous. Not only did the home that Kevin, Joe and Nick Jonas grow up in have an “open-door policy,” meaning that friends and relatives piled in on the holiday, but the siblings…
Continue Reading
-

Even ‘Avatar: Fire and Ash’ can’t lift 2025 box office out of pandemic-crisis doldrums
As “Avatar: Fire and Ash” headed to the big screen this month, theater owners held their breath.
In an uneven year that saw two billion-dollar hits and a viral “chicken jockey” craze, but also a disastrous first quarter and a nearly…
Continue Reading
-

Podcast tours are all the rage: Inside the big business of live shows
On Dec. 4, Amy Poehler hosted a live recording of her less-than-a-year-old podcast, “Good Hang,” at the Fonda Theatre in Hollywood. The crowd wasn’t just packed, it was fully engaged and cheered for pretty much anything (even Poehler’s…
Continue Reading
-
PM to attend WEF’s meeting in Switzerland next month – RADIO PAKISTAN
- PM to attend WEF’s meeting in Switzerland next month RADIO PAKISTAN
- Pakistan PM to attend World Economic Forum’s annual meeting in Switzerland next month Arab News
- Dar directs enhanced engagement strategy for PM’s WEF visit Associated…
Continue Reading
-

From Football Field to Nuclear Safety
Working her way up, Calabria moved to the CNEN at headquarters in Rio de Janeiro as a nuclear safety and radiation protection inspector at nuclear power plants. At the same time, she joined the Brazil branch of Women in Nuclear (WiN), an…
Continue Reading
-

Tameside marathon runner in India says she is battling sickness
A woman who is attempting to run 100 marathons in 100 days across India said she has had to battle the heat and a bout of sickness.
Hannah Cox, who lives on a canal boat in Ashton-under-Lyne, Tameside, ended up in hospital on day 49 of her…
Continue Reading