Cadillac will become F1’s newest arrival in 2026, joining a grid of established teams with rich histories – some more complex than others. From Ferrari’s celebrated roots to the many faces of Mercedes, we delve into the origins of all 11…
Author: admin
-

Tehran makes new threat to Gulf shipping as US says ‘hardest hits’ on Iran ‘yet to come’
‘I’ve never been so scared’: Exhausted passengers land in London from Abu Dhabipublished at 22:22 GMT
Tom Symonds
Reporting from Heathrow Airport Image source, PA Media
Image source, PA MediaImage caption, Jeff and Rebecca Moses, from Manchester
I’m at…
Continue Reading
-
Intelligence assessment warns of Iranian attacks on US following Khamenei's death – Reuters
- Intelligence assessment warns of Iranian attacks on US following Khamenei’s death Reuters
- Ayatollah Ali Khamenei’s assassination will likely backfire. Here is why Al Jazeera
- Inside the plan to kill Ali Khamenei Financial Times
- Months of…
Continue Reading
-

The Shore: Enhanced Edition Celebrates Five Years with a 2026 Xbox Series X|S Launch
Summary
- Coming to Xbox Series X|S this year,
- Dragonis Games’fully optimized Enhanced Edition.
- Five-year milestone since the acclaimed Lovecraftian horror adventure launched 2021.
Five years ago, The Shore introduced PC players to a nightmare…
Continue Reading
-

Sanju Samson: How ‘soul searching’ helped him score match-winning 97 in must-win T20 World Cup match | Cricket News
Sanju Samson said time away from his phone and social media, along with self-reflection, helped him return to form as he scored an unbeaten 97 to guide India into the semifinals of the T20 World Cup 2026.India defeated West Indies by five…
Continue Reading
-

LISHA BAI REFRAMES HERMÈS ON MADISON AVENUE
Artist Lisha Bai’s textiles are studies in depth and dimensionality, built around one simple device: the window. Bai’s two-dimensional, cut-and-stitched works resemble theater sets — small domestic interiors that open onto flattened yet…
Continue Reading
-

Cal Wraps Regular Season At Georgia Tech, Wake Forest
BEAR BITES
• California wraps up the regular season on the road this week, looking for a winning finish to ACC action.
• The Golden Bears (20-9, 8-8 ACC) get their second look at Georgia Tech this season, visiting the Yellow…Continue Reading
-
COVID-19 ARDS survivors face lasting disability and high late mortality, researchers report
Four years after ICU admission, mortality remains strikingly high in ventilated COVID-19 ARDS patients, and many survivors continue to struggle with fatigue, insomnia, functional decline, and reduced quality of life.
Study: Four…
Continue Reading
-

Know where to watch live streaming in India
With PV Sindhu unable to travel for the All England Open Badminton Championships 2026, India’s campaign will be led by Lakshya Sen and the doubles duo of Satwiksairaj Rankireddy–Chirag Shetty in Birmingham from Tuesday.
Badminton matches from…
Continue Reading
-
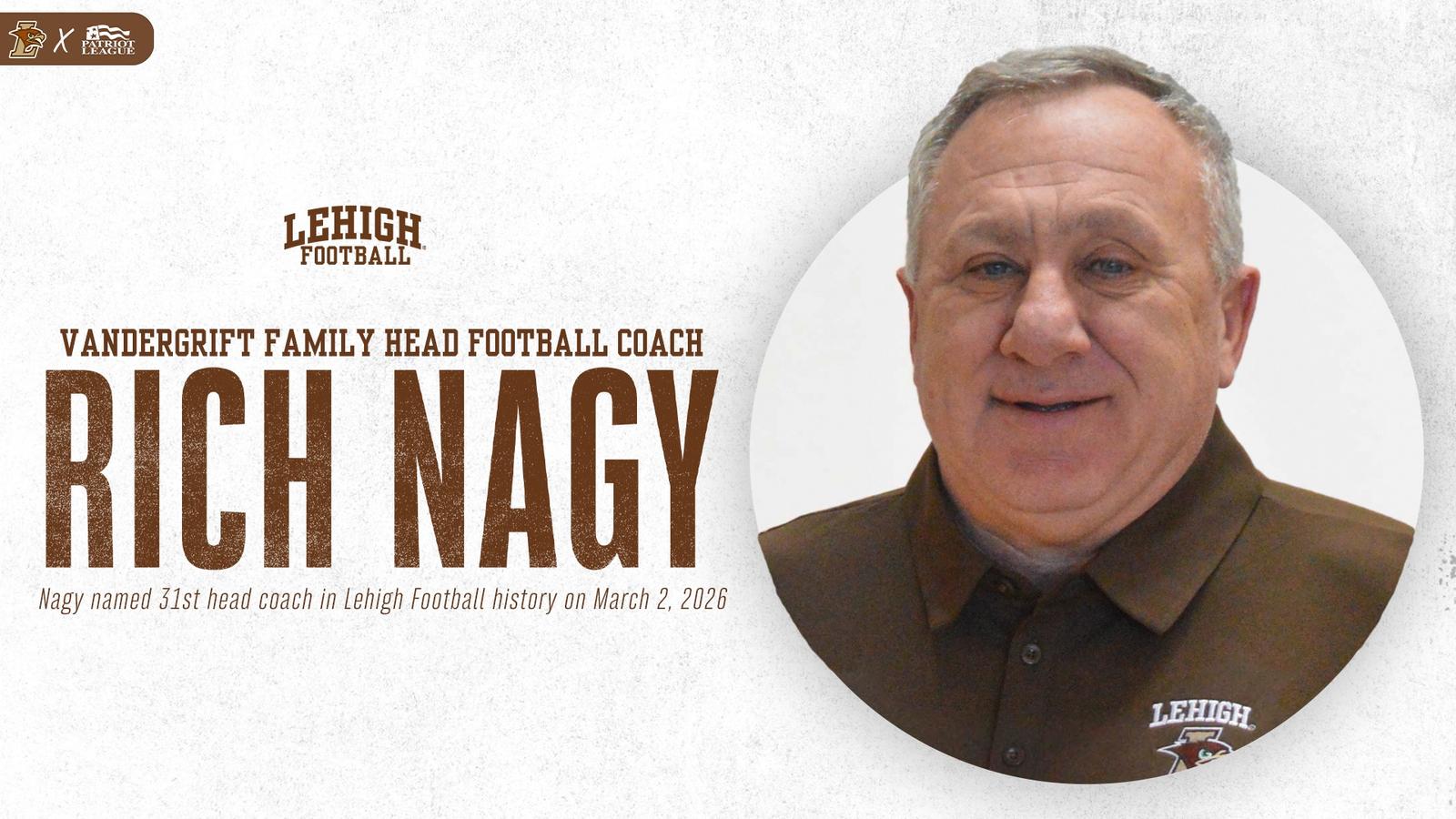
Rich Nagy Elevated to Vandergrift Family Head Football Coach
BETHLEHEM, Pa. – Lehigh University has announced the elevation of Richard Nagy, who will serve as the Vandergrift Family Head Football Coach. Nagy spent the last three years as Lehigh’s Defensive Coordinator, where he led one of the top…Continue Reading
